Abstract
Purpose
Minute T1 colorectal cancer (CRC) lesions (≤5 mm) are rare; however, little is known about their characteristics and aggressiveness. In this study, we evaluated the characteristics of minute T1 CRC in relevance to pathology and treatment.
Methods
This retrospective study included 849 patients with T1 CRC endoscopically or surgically treated between January 2001 and December 2016. The patients were stratified into 4 groups according to tumor size; minute group (≤5 mm), small group (6–10 mm), medium group (11–20 mm), and large group (≥21 mm). Clinicopathological variables were evaluated with respect to tumor size.
Results
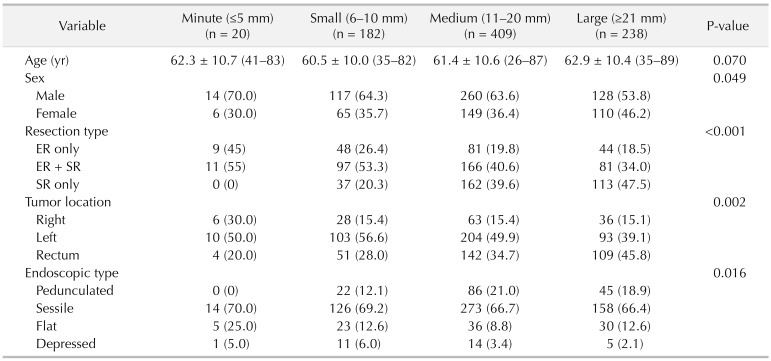
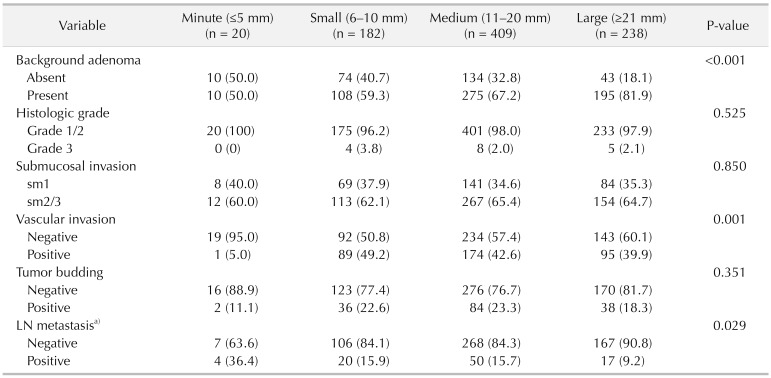
The incidence of the minute T1 CRC was 2.4% (20 of 849). Minute T1 CRC was significantly associated with flat type (minute, 25%; small, 12.6%; medium, 8.8%; large, 12.6%; P = 0.016), right-sided cancer (30%, 15.4%, 15.4%, 15.1%, P = 0.002) and the absence of background adenoma (BGA) (50%, 40.7%, 32.8%, 18.1%, P < 0.001). In patients who underwent surgery, lymph node metastasis (LNM) was significantly higher in the minute group (36.4%, 15.9%, 15.7%, 9.2%, P = 0.029).
As the number of patients undergoing colonoscopy increases, the number of individuals diagnosed with T1 colorectal cancer (CRC) is on the rise. Although the size of T1 CRC lesions varies immensely [1], they are often over 10 mm. However, benignsuspected minute (≤5 mm) colorectal polyps are sometimes proven to be the T1 lesions. Even though it has been previously demonstrated that the tumor size is associated with disease aggressiveness, and negatively correlated with patient survival [2], whether minute T1 CRC lesions are less aggressive than larger T1 CRC lesions remains unknown. Furthermore, the clinicopathological features of the minute T1 CRC lesion have not been elucidated. The aim of this study was to explore the clinical and pathological features of patients with minute T1 CRC.
Our study included 935 T1 CRC patients that underwent endoscopic resection (ER) or surgical resection (SR) at the National Cancer Center, Korea, between January 2001 and December 2016. Patients who had double primary cancer (n = 86) were excluded; thus, 849 patients were included in our analyses (Fig. 1). The clinical and clinicopathological information was acquired from the CRC database and were reviewed retrospectively.
Tumor size was recorded as the maximum horizontal tumor diameter, as measured from formalin-fixed tumor samples. Tumors from cecum to transverse colon were defined as right-sided colon cancers, while tumors from the left colonic flexure to sigmoid colon were defined as left-sided cancers. Tumors located in the rectosigmoid junction or within the rectum were considered as rectal cancers. Tumors were classified into 4 endoscopic types: pedunculated (0-Ip), sessile (0-Is), flat (0-IIa), and depressed (0-IIc), according to the Paris endoscopic classification of superficial neoplastic lesions [3]. In the case of mixed type, 0-IIa + IIc and 0-IIc + IIa were considered as flat and depressed types, respectively.
Background adenoma (BGA) was defined as an adenomatous component microscopically contiguous to resected T1 CRC [4]. The tumors grade was determined according to the World Health Organization criteria [5], so that well-differentiated adenocarcinomas that had glandular structures in >95% of the tumor were classified as grade 1, while moderately differentiated adenocarcinomas with glandular structures in 50%–95% were classified as grade 2. Poorly differentiated adenocarcinomas with <50% glandular structure were classified as grade 3. Grade 3 CRCs, signet ring cell carcinoma and mucinous carcinoma, were considered as high-grade.
In surgically resected specimen, the depth of submucosal (SM) invasion was determined according to the Kudo's classification [6]; as sm1 tumors showed infiltration into the upper third of the SM layer, sm2 tumors had infiltration into the middle third of the SM layer, and sm3 tumors showed infiltration into the lower third of the SM layer. For endoscopically resected sessile or flat tumors, the cut-off limit between sm1 and sm2 was 1,000 µm according to the Paris classification, and the depth of SM invasion exceeding 2,000 µm was defined as sm3. For endoscopically resected pedunculated tumors, the cut-off limit between sm1 and sm2 was the level of the neck, and the depth of SM invasion exceeding 3,000 µm from the neck was defined as sm3 [7]. The sm2 and sm3 tumors were regarded as deep SM invasion. Vascular invasion was defined as the presence of cancer cells within endothelial-lined channels. An isolated cell or a small cluster of fewer than 5 carcinoma cells in the invasive front was defined as a budding focus, with positive tumor budding defined as more than 10 budding foci viewed at 200-fold magnification [8]. According to our previous study, the risk factors for lymph node metastasis (LNM) contain deep SM invasion (sm2 or sm3), vascular invasion, high histological grade, budding [9], and pathological diagnoses were performed by a board-certified pathologist (HJC).
We classified patients into 4 groups according to the size of the tumor: minute group (≤5 mm), small group (6–10 mm), medium group (11–20 mm), and large group (≥21 mm). We then compared the clinicopathological characteristics between the groups. Differences in age, sex, tumor location, endoscopic type, BGA, histologic grade, depth of SM invasion, vascular invasion, and tumor budding were assessed. In radically resected cases (n = 639), the incidence of LNM was also analyzed in each group.
The endoscopic resectability was assessed considering the endoscopic findings, including gross configuration (depression, ulceration, or induration), irregular surface pattern (pit pattern, superficial vascular structure), and nonlifting sign [1011].
Lesions that were considered as endoscopically resectable were treated with primary ER, while primary SR was used for lesions that were determined to be endoscopically unresectable.
Patients that were classified as high risk for LNM after primary ER underwent additional SR. In some cases, transanal excision or transanal endoscopic microsurgery was applied when patients refused SR, or when ER was technically challenging to perform.
Patients were followed-up regularly every 3 months in the first 2 years and every 6 months thereafter. Physical examination, measurement of serum carcinoembryonic antigen, and chest radiography were checked every 3 or 6 months. Abdominopelvic computed tomography was obtained every 6 months. Colonoscopy was performed 1 year postoperatively and then once every 2 years.
Confirmation of recurrence was based on imaging or pathologic findings. Recurrence-free survival (RFS) as an oncologic outcome was analyzed in each treatment group (ER vs. ER, additional SR vs. SR only). RFS was calculated as the time from the primary resection until patient recurrence or death.
Patient demographics and clinical characteristics were analyzed using the Student t-test for continuous variables and cross-table analysis using Fisher exact test or the chisquare test, following statistical validity. RFS was analyzed using the Kaplan-Meier method. P-value <0.05 was considered statistically significant for all analyses.
Out of 849 T1 CRC patients, 182 patients underwent ER only, 355 patients ER plus additional SR, and 312 patients SR only. The mean tumor size was 18.0 mm (range, 3.5–115 mm). There were 20 cases of the minute group (2.4%), 182 of the small group (21.4 %), 409 of the medium group (48.2%), and 238 of the large group (28.0%) (Fig. 1). The characteristics of all 849 patients are summarized in Table 1.
Patients of the minute group exhibited higher proportion of flat type tumors (minute, 25%; small, 12.6%; medium, 8.8%; large, 12.6%; P = 0.016), and right-sided tumors (minute, 30%; small, 15.4%; medium, 15.4%; large, 15.1%; P = 0.002). The clinical characteristics of the patients of each group are detailed in Table 2.
The patients of the minute group exhibited higher proportion of BGA absence (minute, 50%; small, 40.7%; medium, 32.8%; large, 18.1%; P < 0.001). Moreover, patients of, the minute group demonstrated significantly lower incidence of vascular invasion (minute, 5%; small, 49.2%; medium, 42.6%; large, 39.9%; P = 0.001). In surgically resected patients, the higher incidence of LNM was noted in the minute group (36.4%, 15.9%, 15.7%, 9.2%, P = 0.029). The histologic grade, depth of SM invasion, and tumor budding did not differ significantly between the groups (Table 3).
From the 20 patients of the minute group, none had pedunculated type CRC, while 14 (70.0%), 5 (25.0%), and 1 (5.0%) had sessile type, flat type, and depressed type tumors, respectively (Fig. 2). All 20 patients underwent primary ER; 11 patients underwent additional SR. Out of the 11 patients that underwent additional SR, 4 had LNM. Out of 9 patients who underwent ER only, 3 patients showed unfavorable histologic factors for LNM and refused additional SR. Further details are shown in Supplementary Table 1.
The median follow-up period was 59 months (range, 0–167 months). Thirty-six patients experienced local recurrence or distant metastasis (ER, 12; ER + additional SR, 10; SR, 14). For the ER, ER + additional SR, SR groups, recurrence rates were 6.6%, 2.8%, and 4.5%, respectively (P = 0.114), while the 5-year RFS rates were 95.6%, 97.7%, and 96.9%, respectively (Fig. 3) (P = 0.111). The recurrence rate and RFS were not significantly different between the different treatment groups.
Larger colorectal polyp size has been associated with increased malignancy. T1 CRC lesions are usually over 10 mm in size, the vast majority of them being over 5 mm. Benign-suspected minute (≤5 mm) colorectal polyps are rarely diagnosed as T1 cancer lesions after ER, but the characteristics of minute CRC have not been established yet.
Our study indicated that the incidence of minute lesions in T1 CRCs was 2.4%, and that minute T1 CRCs exhibited a higher proportion of flat type and right-sided cancers. These results suggest that the minute T1 CRC lesions are likely not to be diagnosed. Nonpolypoid colorectal neoplasms are not easily detected by colonoscopy since the subtle findings can be hard to differentiate from those of normal mucosa [1213]. Additionally, the right-sided location might also contribute to nondiagnosis, especially in cases of incomplete colonoscopic examination.
Interval CRC is defined as CRC diagnosed after colonoscopy in which no cancer is found, and before the next recommended examination [14]. Previous studies have reported that there are more interval cancers in the right colon than in the left colon [1516]. Chen et al. [17] have demonstrated that interval cancers were common in males over 50 years of age. According to our findings, minute T1 CRC was more frequent in the right colon and male patients, suggesting that interval CRCs may originate from the minute T1 CRC lesions.
CRCs without BGA are regarded as de novo cancers [1819]. In our previous study, the absence of BGA was identified as one of the predictors of LNM in T1 CRC [20]. Our findings showed that minute T1 CRC is associated with the absence of BGA and that the incidence of LNM is significantly higher in patients with minute T1 CRCs, which may imply that minute T1 CRC lesions might be more aggressive than the larger T1 CRC lesions.
On the other hand, we found that minute T1 CRC lesions exhibited a significantly lower incidence of vascular invasion. Although deep SM invasion has been identified as one of the predictive factors for LNM [2122], not many studies have addressed the relationship between tumor size and SM invasion. Ikehara et al. [23] reported that no significant association between tumor size and deep SM invasion, yet invasive pit pattern was correlated with SM invasion in sessile and superficial cancers. Similarly, deep SM invasion was not significantly associated with tumor size in our study.
This study had some limitations. Importantly, although we analyzed a relatively large patient cohort, the study was limited by the small number of the minute T1 CRC cases. Future studies involving a larger cohort is needed. Moreover, we did not perform a molecular characterization of the tumors. Lastly, the tumor sizes were determined from formalin-fixed tumor specimens, which likely did not accurately represent the tumor size in the patients.
In conclusion, minute T1 CRCs were significantly associated with flat type, right-sided cancer, as well as with the absence of BGA and LNM. These results suggested that minute T1 CRC lesions are often aggressive and are likely not to be found. Thus, endoscopists should pay particular attention to patients with CRC lesions, not to miss the minute flat lesion, especially in the right colon.
Notes
References
1. Togashi K, Utano K, Kijima S, Sato Y, Horie H, Sunada K, et al. Laterally spreading tumors: l imitations of computed tomography colonography. World J Gastroenterol. 2014; 20:17552–17557. PMID: 25516670.
2. Kornprat P, Pollheimer MJ, Lindtner RA, Schlemmer A, Rehak P, Langner C. Value of tumor size as a prognostic variable in colorectal cancer: a critical reappraisal. Am J Clin Oncol. 2011; 34:43–49. PMID: 20101166.
3. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003; 58(6 Suppl):S3–S43. PMID: 14652541.
4. Jass JR. Do all colorectal carcinomas arise in preexisting adenomas? World J Surg. 1989; 13:45–51. PMID: 2658353.

5. Kleihues P, Cavenee WK. International Agency for Research on Cancer. Pathology and genetics of tumours of digestive system. Lyon: IARC Press;2000. (World Health Organization classification of tumours; v. 2).
6. Kudo S. Endoscopic mucosal resection of flat and depressed types of early colorectal cancer. Endoscopy. 1993; 25:455–461. PMID: 8261988.

7. Kitajima K, Fujimori T, Fujii S, Takeda J, Ohkura Y, Kawamata H, et al. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol. 2004; 39:534–543. PMID: 15235870.

8. Ueno H, Price AB, Wilkinson KH, Jass JR, Mochizuki H, Talbot IC. A new prognostic staging system for rectal cancer. Ann Surg. 2004; 240:832–839. PMID: 15492565.

9. Ha RK, Han KS, Sohn DK, Kim BC, Hong CW, Chang HJ, et al. Histopathologic risk factors for lymph node metastasis in patients with T1 colorectal cancer. Ann Surg Treat Res. 2017; 93:266–271. PMID: 29184880.

10. Kobayashi Y, Kudo SE, Miyachi H, Hosoya T, Ikehara N, Ohtsuka K, et al. Clinical usefulness of pit patterns for detecting colonic lesions requiring surgical treatment. Int J Colorectal Dis. 2011; 26:1531–1540. PMID: 21607587.

11. Uno Y, Munakata A. The non-lifting sign of invasive colon cancer. Gastrointest Endosc. 1994; 40:485–489. PMID: 7926542.

12. Soetikno RM, Kaltenbach T, Rouse RV, Park W, Maheshwari A, Sato T, et al. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. JAMA. 2008; 299:1027–1035. PMID: 18319413.

13. Heresbach D, Barrioz T, Lapalus MG, Coumaros D, Bauret P, Potier P, et al. Miss rate for colorectal neoplastic polyps: a prospective multicenter study of back-to-back video colonoscopies. Endoscopy. 2008; 40:284–290. PMID: 18389446.

14. Benedict M, Galvao Neto A, Zhang X. Interval colorectal carcinoma: an unsolved debate. World J Gastroenterol. 2015; 21:12735–12741. PMID: 26668498.

15. Bressler B, Paszat LF, Chen Z, Rothwell DM, Vinden C, Rabeneck L. Rates of new or missed colorectal cancers af ter colonoscopy and thei r r isk factors: a population-based analysis. Gastroenterology. 2007; 132:96–102. PMID: 17241863.
16. Singh S, Singh PP, Murad MH, Singh H, Samadder NJ. Prevalence, risk factors, and outcomes of interval colorectal cancers: a systematic review and meta-analysis. Am J Gastroenterol. 2014; 109:1375–1389. PMID: 24957158.

17. Chen TH, Yen MF, Lai MS, Koong SL, Wang CY, Wong JM, et al. Evaluation of a selective screening for colorectal carcinoma: the Taiwan Multicenter Cancer Screening (TAMCAS) project. Cancer. 1999; 86:1116–1128. PMID: 10506694.
18. Kanazawa T, Watanabe T, Nagawa H. Does early polypoid colorectal cancer with depression have a pathway other than adenoma-carcinoma sequence? Tumori. 2003; 89:408–411. PMID: 14606645.

19. Mueller JD, Bethke B, Stolte M. Colorectal de novo carcinoma: a review of its diagnosis, histopathology, molecular biology, and clinical relevance. Virchows Arch. 2002; 440:453–460. PMID: 12021919.

20. Han KS, Lim SW, Sohn DK, Chang HJ, Oh JH, Lee JH, et al. Clinicopathological characteristics of T1 colorectal cancer without background adenoma. Colorectal Dis. 2013; 15:e124–e129. PMID: 23294594.

21. Nascimbeni R, Burgart LJ, Nivatvongs S, Larson DR. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum. 2002; 45:200–206. PMID: 11852333.

22. Nivatvongs S, Rojanasakul A, Reiman HM, Dozois RR, Wolff BG, Pemberton JH, et al. The risk of lymph node metastasis in colorectal polyps with invasive adenocarcinoma. Dis Colon Rectum. 1991; 34:323–328. PMID: 1848810.

23. Ikehara H, Saito Y, Matsuda T, Uraoka T, Murakami Y. Diagnosis of depth of invasion for early colorectal cancer using magnifying colonoscopy. J Gastroenterol Hepatol. 2010; 25:905–912. PMID: 20546444.

SUPPLEMENTARY MATERIAL
Supplementary Table 1 can be found via
https://doi.org/10.4174/astr.2020.98.4.199.
Supplementary Table 1
Clinicopathological characteristics of 20 patients with minute T1 colorectal cancer
Fig. 1
Schematic representation of the patient grouping according to tumor size. CRC, colorectal cancer.
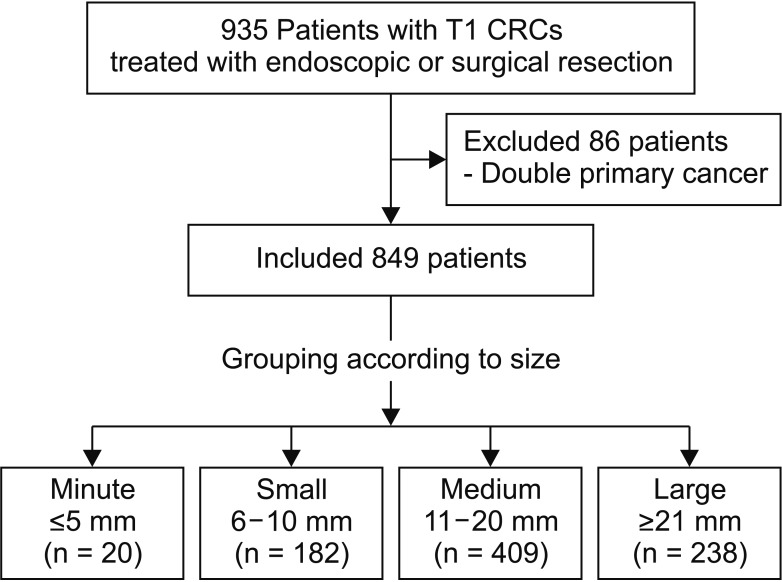
Fig. 2
Representative images of endoscopic findings in patients with minute T1 colorectal cancer. Sessile type (A), flat type (B), and depressed type (C).
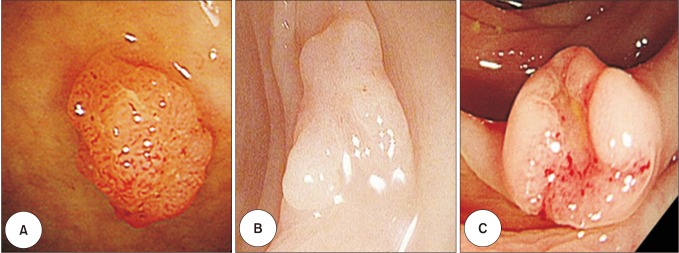
Fig. 3
Recurrence-free survival in colorectal cancer patients according to the treatment method. ER, endoscopic resection; SR, surgical resection.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download