Abstract
Purpose
Since the treatment strategy for benign and malignant pancreatic lesions differ, we aimed to evaluate the clinical value of PET/CT in the diagnosis and management of pancreatic lesions.
Methods
Ninety patients who had a histologically confirmed pancreatic lesion were studied. Receiver operating characteristic (ROC) curve analysis was used to investigate the ability of PET/CT to differentiate malignant lesions from benign tumors.
Results
The malignant and benign groups comprised 64 and 26 patients, respectively. Despite the similarity in the size of primary tumors (P = 0.588), the mean maximum standardized uptake values (SUVmax) obtained from PET/CT imaging were significantly higher in malignant lesions (9.36 ± 5.9) than those of benign tumors (1.04 ± 2.6, P < 0.001). ROC analysis showed that the optimal SUVmax cutoff value for differentiating malignant lesions (to an accuracy of 91%; 95% confidence interval, 83%–98%) from benign tumors was 3.9 (sensitivity, 92.2%; specificity, 84.6%).
Go to : 
Despite advances in imaging techniques, the accurate diagnosis of pancreatic lesions remains challenging. As treatment modalities differ based on the nature of the lesion, determining whether a lesion is benign or malignant is crucial. Moreover, the histopathologic features and localization of malignant pancreatic lesions have prognostic significance [123]. The maximum standardized uptake value (SUVmax) is an indicator of glucose metabolism of tumor cells identified by [18F]-fluorodeoxyglucose (18F-FDG) PET/CT. In addition to the ability to demonstrate tumor aggressiveness, SUVmax may offer an advanced method of depicting pancreatic pathologies on the basis of the localized uptake of FDG-tagged glucose by cancerous cells [4567].
As pancreatic surgery is involved with high morbidity and mortality (5%–9%), the potential benefit of PET/CT evaluation when formulating a treatment strategy deserves to be examined [6789]. PET/CT imaging of pancreatic lesions may help guide the decision-making process and avoid unnecessary surgeries. Several studies have reported the clinical value of PET/CT in the identification and management of pancreatic lesions. Although encouraging results have been shown, the function of PET/CT in the diagnosis of pancreatic lesions is not well-defined [678910].
In this study, we aimed to evaluate the ability of PET/CT imaging to differentiate between benign and malignant pancreatic lesions with a high degree of sensitivity and specificity by determining the SUVmax threshold.
Go to : 
From June 2009 to June 2018, 90 patients who had a histologically confirmed pancreatic lesion, and received fullbody 18F-FDG PET/CT scans in our hospital, were included into the study. Diabetic patients were not included in the study based on the fact that the impact of untagged blood glucose on 18F-FDG uptake is much stronger in organs such as the brain that have prominent glucose metabolism, though the fundamental process of this association is not thoroughly apprehended [11]. This study was approved by the Institutional Review Board of Cukurova University Faculty of Medicine (IRB No. 06.02.2015/39/17), and written informed permission was taken from every patient who registered into the study.
In addition to the patients' demographic characteristics, the median diameter and localization of pancreatic lesions on preoperative CT and MRI were recorded. All surgical procedures and microscopic examination of both surgical and fine-needle aspiration biopsies were done by the same surgery and pathology staff. All patients' histopathological findings were reviewed and classified as benign or malignant. The histopathologic type, metastatic lymph node count, and presence of remote metastasis were established for malignant lesions.
The patients were instructed to abstain from food for a minimum 4 hours before the imaging. Blood glucose levels were quantified to confirm that all serum glucose levels were under 144 mg/dL before injection of 18F-FDG intravenously. Approximately 370 MBq (10 mCi) of 18F-FDG per patient was administered through the antecubital vein. Patients weighing above 70 kg were administered a dose of 444 MBq (12 mCi) 18F-FDG. Patients rested in a room for one hour after which a full-body imaging was taken. Whole body 18F-FDG PET/CT scans were obtained on a Biograph PET/CT system (Siemens Molecular Imaging, Hoffman Estates, IL, USA). The system consists of a full ring dedicated PET and a 2-slice spiral CT. Data were analyzed on an image fusion workstation (Symbia; Siemens Healthcare Sector, Erlangen, Germany). The administered activity, time of administration, and patient body weight were used for calculation of SUVmax.
Statistical analysis was performed using IBM SPSS Statistics ver. 24.0 (IBM Co., Armonk, NY, USA). If continuous variables were normally distributed, they were presented as the mean ± standard deviation (P > 0.05 on the Kolmogorov-Smirnov test), otherwise they were presented as the median value.
Comparisons between groups were performed using Student t-test for normally distributed data and the Mann-Whitney U-test for nonnormally distributed data. The differences in categorical variables between the groups were analyzed using the chi-square test. Receiver operating characteristic (ROC) curves were constructed, and the area under the curve (AUC), sensitivity, and specificity were calculated. Statistical significance was predetermined at P < 0.05.
Go to : 
A whole of 90 patients who met the enrollment criteria were analyzed. The mean age of the patients was 54.4 ± 11.4 years and a female predominance was apparent (n = 53, 58.9%). The patient demographic characteristics and operative data are outlined in Table 1. Despite similarity in the diameter homogeneity of the primary tumors (malignant: 2.4 ± 0.6 vs. benign: 2.5 ± 0.6; P = 0.936), the median SUVmax value acquired from PET/CT imaging was significantly higher in patients with malignant lesions (median SUVmax: 7.5 [0–30.1]) compared to those with benign tumors (median SUVmax: 0 [0–9.3]; P < 0.001).
Table 2 presents the distribution of patients with pancreatic lesions based on histopathological reports. Of 90 patients enrolled, 64 were in the malignant group. The most common malignant histopathology was adenocarcinoma (n = 43) followed by neuroendocrine tumor (n = 13) and non-Hodgkin lymphoma (n = 6).
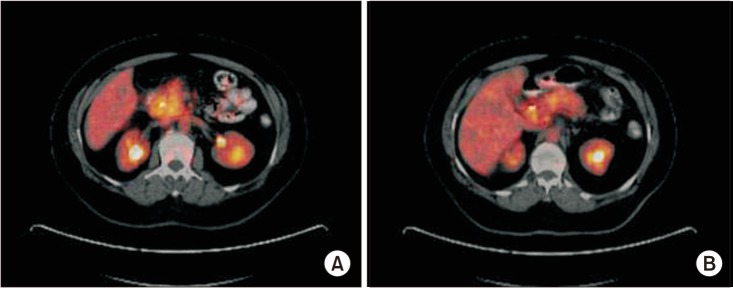
Despite having benign pathologies, one patient with mucinous cystadenoma (SUVmax: 3.10) and 3 with chronic pancreatitis (SUVmax: 7.95 [Fig. 1A], 8.2, and 9.3 [Fig. 1B], respectively) had elevated SUVmax values on PET/CT imaging. A positive association between tumor size and SUVmax value was detected in the malignant group (r = 0.49; P < 0.001). However, there was no relationship between the tumor location and SUVmax value (P = 0.717) for malignant pancreatic lesions. Similarly, no correlation between histopathological features and SUVmax values was observed in malignant pancreatic lesions (P = 0.051).
Subgroup analysis of the malignant group revealed that the SUVmax values acquired from the primary origin of metastatic pancreatic tumors were not significantly different from those of nonmetastatic tumors (median [range]: 8.5 [0.0–25.5] vs. 6.0 [3.1–30.1]; P = 0.066). The ROC-test showed that the most favorable cutoff limit for SUVmax to differentiate malignant pancreatic lesions from benign tumors (to an accuracy of 91%; 95% confidence interval, 83%–98%) was 3.9 (sensitivity, 92.2%; specificity, 84.6%). The AUC was estimated at 0.91 (Fig. 2).
Go to : 
Although there is an increasing interest in precisely differentiating malignant lesions from their benign counterparts, conventional radiological imaging modalities, including CT, MRI, and endoscopic ultrasonography, have been considered to be insufficient for developing an accurate treatment algorithm. With its ability to elucidate the whole body's functional and anatomic imaging features [456], the function of PET/CT in characterizing pancreatic lesions was investigated in the current study, revealing that PET/CT can differentiate malignant lesions from benign tumors with high sensitivity and specificity.
Cancer of the pancreas is the fourth major cause of cancer-associated death with an approximately 5-year overall survival ratio of 20%. Furthermore, only 10%–30% of patients diagnosed with pancreatic cancer are eligible for resection, and life expectancy is limited to months in those with locally advanced and metastatic disease. Thus, prompt and correct diagnosis is of paramount importance for general survival and life quality in patients with pancreatic cancers. Kim et al. [12] investigated survival outcome and prognostic factors of neoadjuvant treatment followed by resection for borderline resectable pancreatic cancer. When prognostic factors for survival were analysed, they reported that, in univariate analyses, partial remission in the neoadjuvant setting reduced CA 19-9 level and pancreatectomy were associated with a better outcome. In multivariate analyses, partial remission after neoadjuvant therapy and pancreatectomy were independent variables affecting outcomes.
PET/CT is an imaging method dependent upon detecting increased glucose intake, a differentiating property in the majority of malignant tumors that is partly connected with the overexpression of glucose transporter-1 and increased hexokinase function [1314]. Since adenomas are benign pathologies, few patients are eager to undergo 18F-FDG. Previous studies have already shown that an FDG whole body PET scan is more accurate than conventional imaging techniques in the differentiation of cystic pancreatic lesions, though conflicting results have also been published in the medical literature. In this context, Mansour et al. [15] evaluated 68 patients with whole body PET imaging for cystic lesion of the pancreas. They reported that, within the group of resected patients, the sensitivity of PET for identifying malignant pathology was 57%, and the specificity was 85%. The sensitivity and specificity of PET for malignancy in this study was lower than previously reported, and PET findings did not identify otherwise occult malignant cysts. They stated that they did not believe whole body FDG-PET to be essential in the evaluation of cystic lesions of the pancreas. However, Kauhanen et al. [16] reported that the diagnostic accuracy was 94% for 18F-FDG PET/CT in the differential diagnosis of malignant and benign pancreatic cysts. 18F-FDG PET/CT had a negative predictive value of 100% and a positive predictive value of 75% for pancreatic cysts. Current guidelines for the management of mucinous cystic neoplasia are based on the assumption that these lesions can be classified correctly on the basis of imaging features. Sato et al. [17] reported a case of a 35-year-old woman with mucinous cystic neoplasia which showed inhomogeneous FDG accumulation in its cyst wall, and maximum SUVmax 1- and 2-hour postinjection were 2.83 and 3.51, respectively. The epithelium was focally denuded and ovarian-like stroma with macrophage migration, which phagocytosed red blood cells, and fibrosis were recognized on histopathological examination. These histopathological findings suggested that FDG accumulates not in the monolayer epithelium but in ovarian-like stroma with macrophage migration and fibrosis. They speculated that macrophage migration and fibrosis were considered to have contributed to FDG accumulation in this mucinous cystic neoplasm. In our study, one patient with mucinous cystic neoplasia had false-positive FDG accumulation, and this state was suggestive of thickening of the cyst wall.
It has been reported that increased FDG collection might be utilized as an indicator of inflammatory processes [1819]. However, polymorphonuclear neutrophils, macrophages and other inflammatory cells, which highly express glucose transporter and glycolytic enzymes, can also lead to local accumulation of FDG. This condition makes it difficult to distinguish pancreatic cancer from acute or chronic pancreatitis, pancreatic tuberculosis, and autoimmune pancreatic diseases. A previous study has shown that the FDG SUVmax value of a tumor was generally higher than 4.0 in pancreatic cancer patients, 3.0–4.0 in patients with chronic pancreatitis, and under 3.0 in otherwise healthy individuals [20]. Our results corroborated these findings; however, considering that all 3 patients with chronic pancreatitis had a SUVmax value higher than 7, we recommend that clinicians be aware of possible exceptional situations when evaluating a high SUVmax value.
There is quite little data concerning the diagnostic function of PET/CT in patients with pancreatic neoplasia in the current literature. Several studies have ascertained the FDG-sensitivity of pancreatic lesions, and revealed some SUVmax cutoff values ranging from 2.5 to 3.6. No standardized threshold of SUVmax in differentiating benign lesions from malignant tumors has been reported in the medical literature so far. Sperti et al. [21] investigated 50 patients with suspected cystic tumor of the pancreas or intraductal papillary mucinous tumors by using 18-FDG. Seventeen patients had malignant cystic lesions. Sixteen (94%) showed increased 18-FDG uptake (SUV > 2.5), including 2 patients with carcinoma in situ. They found that sensitivity, specificity, positive and negative predictive value, and accuracy of 18-FDG PET in detecting malignant tumors were 94%, 94%, 89%, 97%, and 94%, respectively. Tomimaru et al. [22] studied 29 patients with FDG-PET in preoperative differentiation of benign and malignant intraductal papillary mucinous neoplasms (IPMNs) of the pancreas. The specificity, sensitivity and accuracy values were best for an SUVmax of 2.5 (100%, 93%, and 96%, respectively). The SUVmax of 2.5 offered the best diagnosis of malignant IPMNs. However, Kauhanen et al. [16] examined 31 patients with 18F-FDG PET/CT in differentiating malignant from benign pancreatic cysts. They reported that, when the SUVmax value was set at 3.6, the sensitivity and specificity of 18F-FDG PET/CT were 100% and 88%, respectively. Our study presents a SUVmax cutoff value of 3.9 for differentiating pancreatic tumors with high sensitivity and specificity rates (92.2% and 84.6%, respectively). Equivocal findings on advanced imaging modalities can be further elucidated by PET/CT imaging in pancreatic neoplasia.
When a pancreatic lesion is diagnosed, the determination of whether the tumor is resectable is crucial since surgical resection constitutes the main component of treatment. The available diagnostic imaging methods are restricted in their capability to identify small (<2 cm) pancreatic lesions [2324]. While our findings demonstrated a high success rate in terms of detection, clinically significant SUVmax values were only correlated with increasing diameter of malignant tumors on PET/CT imaging. However, we did not find any association between the location of malignant tumors within the pancreas and the SUVmax value.
There were some limitations of our study. Firstly, our study group had a small number of patients. In our hospital, PET/CT is not routinely used for preoperative evaluation of pancreatic lesions. There were also a limited number of eligible patients whose data were sufficient for our study. Secondly, we had a heterogenous patient population which included different types of tumor pathology and tumor localizations. We had to reach some results without separating them due to the number of patients. Thirdly, despite all patients having PET/CT images, some patients had only CT images, MRI images or both. While some patients were able to be compared with 3 imaging modalities, others could be compared between PET/CT and existing radiological methods.
In conclusion, PET/CT evaluation of pancreatic lesions confers advantages including precise assessment of malignant potential using a SUVmax threshold of 3.9. Increased SUVmax value was found to be associated with the diameter and malignant features of the lesions but not with their intrapancreatic location. Further studies are needed to support our findings.
Go to : 
Notes
Author Contribution:
Conceptualization: ATA, ZT, IBG, AR.
Formal Analysis: ATA, ZT, AGS, AU, IBG, AR.
Investigation: ATA, ZT, AGS, AU, IBG, AR.
Methodology: ATA, ZT, AGS, AU, IBG, AR.
Project Administration: ATA, ZT, AGS, AU, IBG, AR.
Writing — Original Draft: ATA, ZT, IBG, AR.
Writing — Review & Editing: ATA, ZT, IBG, AR.
Go to : 
References
1. Sener SF, Fremgen A, Menck HR, Winchester DP. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985-1995, using the National Cancer Database. J Am Coll Surg. 1999; 189:1–7. PMID: 10401733.
2. Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004; 363:1049–1057. PMID: 15051286.

3. Varadhachary GR, Tamm EP, Abbruzzese JL, Xiong HQ, Crane CH, Wang H, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006; 13:1035–1046. PMID: 16865597.

4. Kauhanen SP, Komar G, Seppanen MP, Dean KI, Minn HR, Kajander SA, et al. A prospective diagnostic accuracy study of 18F-fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Ann Surg. 2009; 250:957–963. PMID: 19687736.

5. Okamoto K, Koyama I, Miyazawa M, Toshimitsu Y, Aikawa M, Okada K, et al. Preoperative 18[F]-fluorodeoxyglucose positron emission tomography/computed tomography predicts early recurrence after pancreatic cancer resection. Int J Clin Oncol. 2011; 16:39–44. PMID: 20862596.

6. Nakamoto Y, Higashi T, Sakahara H, Tamaki N, Kogire M, Doi R, et al. Delayed (18)F-fluoro-2-deoxy-D-glucose positron emission tomography scan for differentiation between malignant and benign lesions in the pancreas. Cancer. 2000; 89:2547–2554. PMID: 11135214.
7. Diederichs CG, Staib L, Vogel J, Glasbrenner B, Glatting G, Brambs HJ, et al. Values and limitations of 18F-fluorodeoxyglucose-positron-emission tomography with preoperative evaluation of patients with pancreatic masses. Pancreas. 2000; 20:109–116. PMID: 10707924.

8. Delbeke D, Rose DM, Chapman WC, Pinson CW, Wright JK, Beauchamp RD, et al. Optimal interpretation of FDG PET in the diagnosis, staging and management of pancreatic carcinoma. J Nucl Med. 1999; 40:1784–1791. PMID: 10565771.
9. Hosten N, Lemke AJ, Wiedenmann B, Bohmig M, Rosewicz S. Combined imaging techniques for pancreatic cancer. Lancet. 2000; 356:909–910. PMID: 11036898.

10. Beyer T, Townsend DW, Brun T, Kinahan PE, Charron M, Roddy R, et al. A combined PET/CT scanner for clinical oncology. J Nucl Med. 2000; 41:1369–1379. PMID: 10945530.
11. Busing KA, Schonberg SO, Brade J, Wasser K. Impact of blood glucose, diabetes, insulin, and obesity on standardized uptake values in tumors and healthy organs on 18F-FDG PET/CT. Nucl Med Biol. 2013; 40:206–213. PMID: 23228852.
12. Kim HS, Jang JY, Han Y, Lee KB, Joo I, Lee DH, et al. Survival outcome and prognostic factors of neoadjuvant treatment followed by resection for borderline resectable pancreatic cancer. Ann Surg Treat Res. 2017; 93:186–194. PMID: 29094028.

13. Higashi T, Saga T, Nakamoto Y, Ishimori T, Mamede MH, Wada M, et al. Relationship between retention index in dual-phase (18)F-FDG PET, and hexokinase-II and glucose transporter-1 expression in pancreatic cancer. J Nucl Med. 2002; 43:173–180. PMID: 11850481.
14. Kitasato Y, Yasunaga M, Okuda K, Kinoshita H, Tanaka H, Okabe Y, et al. Maximum standardized uptake value on 18F-fluoro-2-deoxy-glucose positron emi ssion tomog raphy/computed tomography and glucose transporter-1 expression correlates with survival in invasive ductal carcinoma of the pancreas. Pancreas. 2014; 43:1060–1065. PMID: 25121413.
15. Mansour JC, Schwartz L, Pandit-Taskar N, D'Angelica M, Fong Y, Larson SM, et al. The utility of F-18 fluorodeoxyglucose whole body PET imaging for determining malignancy in cystic lesions of the pancreas. J Gastrointest Surg. 2006; 10:1354–1360. PMID: 17175454.

16. Kauhanen S, Rinta-Kiikka I, Kemppainen J, Gronroos J, Kajander S, Seppanen M, et al. Accuracy of 18F-FDG PET/CT, multidetector CT, and MR imaging in the diagnosis of pancreatic cysts: a prospective single-center study. J Nucl Med. 2015; 56:1163–1168. PMID: 26045314.

17. Sato M, Hiyama T, Kato K, Shirasu T, Yoshimi F, Nagai H, et al. F-18 FDG accumulation in mucinous cystic neoplasm of pancreas. Clin Nucl Med. 2011; 36:45–48. PMID: 21157210.

18. Shreve PD. Focal fluorine-18 fluorodeoxyglucose accumulation in inflammatory pancreatic disease. Eur J Nucl Med. 1998; 25:259–264. PMID: 9580859.

19. Balink H, Tan SS, Veeger NJ, Holleman F, van Eck-Smit BL, Bennink RJ, et al. 18F-FDG PET/CT in inflammation of unknown origin: a cost-effectiveness pilot-study. Eur J Nucl Med Mol Imaging. 2015; 42:1408–1413. PMID: 25655485.
20. Imdahl A, Nitzsche E, Krautmann F, Hogerle S, Boos S, Einert A, et al. Evaluation of positron emission tomography with 2-[18F]fluoro-2-deoxy-D-glucose for the differentiation of chronic pancreatitis and pancreatic cancer. Br J Surg. 1999; 86:194–199. PMID: 10100786.

21. Sperti C, Pasquali C, Decet G, Chierichetti F, Liessi G, Pedrazzoli S. F-18-fluorodeoxyglucose positron emission tomography in differentiating malignant from benign pancreatic cysts: a prospective study. J Gastrointest Surg. 2005; 9:22–28. PMID: 15623441.

22. Tomimaru Y, Takeda Y, Tatsumi M, Kim T, Kobayashi S, Marubashi S, et al. Utility of 2-[18F] fluoro-2-deoxy-D-glucose positron emission tomography in differential diagnosis of benign and malignant intraductal papillary-mucinous neoplasm of the pancreas. Oncol Rep. 2010; 24:613–620. PMID: 20664965.

23. Akita H, Takahashi H, Ohigashi H, Tomokuni A, Kobayashi S, Sugimura K, et al. FDG-PET predicts treatment efficacy and surgical outcome of pre-operative chemoradiation therapy for resectable and borderline resectable pancreatic cancer. Eur J Surg Oncol. 2017; 43:1061–1067. PMID: 28389044.

24. Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007; 297:267–277. PMID: 17227978.
Go to : 




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download