Abstract
Purpose
Intrahepatic recurrence has a significant effect on the survival of hepatocellular carcinoma (HCC) patients. We aimed to determine if there are useful indicators in predicting the recurrence of liver cancer after a hepatic resection.
Methods
We retrospectively reviewed medical records of 210 HCC patients who underwent hepatectomy between January 2009 and December 2015. We examined clinic-pathological variables comparing 2 groups of HCC patients, either intrahepatic recurrence or not.
Results
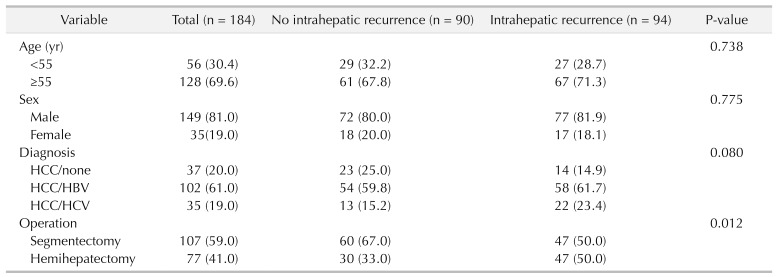
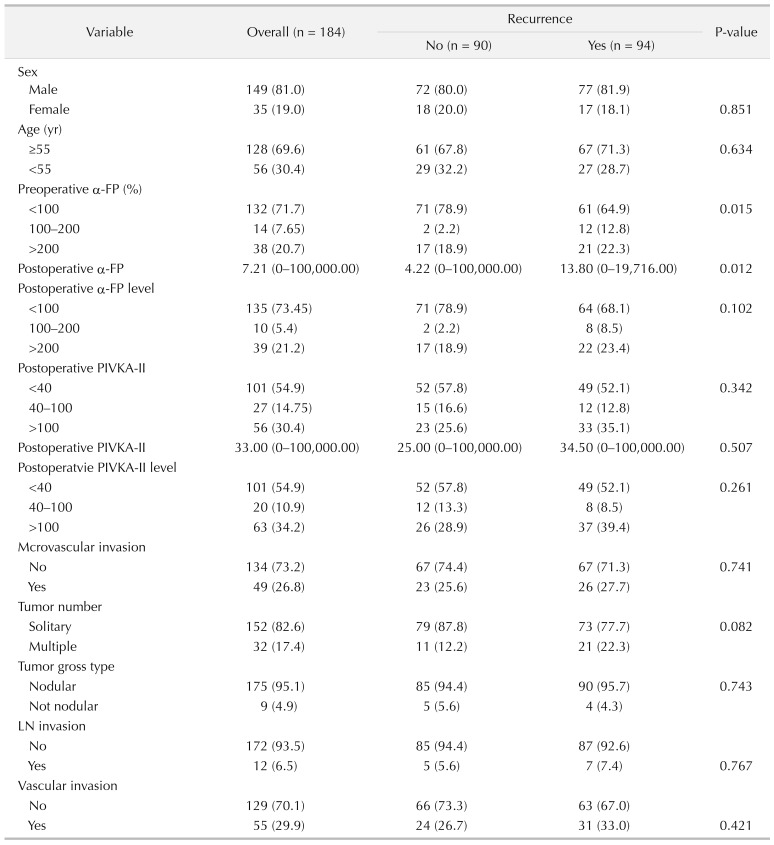
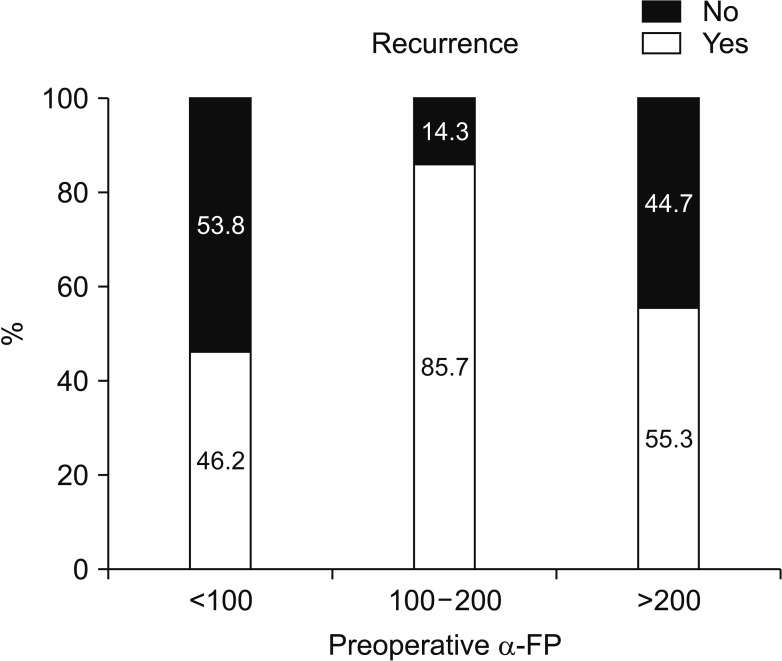
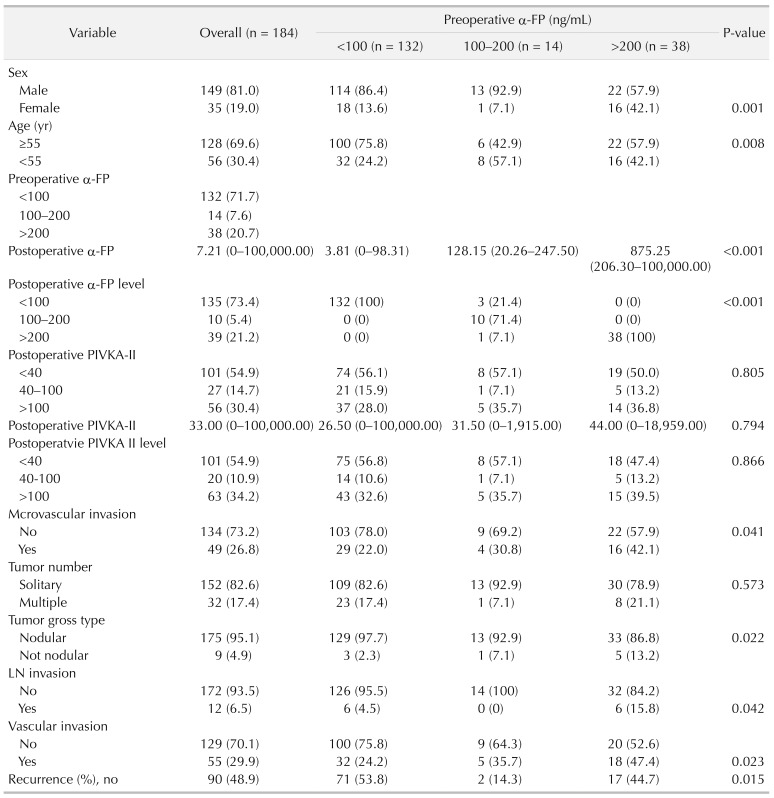
We divided 184 patients into 2 groups; 94 patients (51.1%) with intrahepatic recurrence and 90 patients (48.9%) without intrahepatic recurrence. Multivariate analysis showed operation type, preoperative α-FP, postoperative protein induced by vitamin K absence-II (PIVKA-II) elevation, and multiple tumor number were closely associated with intrahepatic recurrence. The preoperative PIVKA-II level was not statistically significant in postoperative intrahepatic recurrence rate. The recurrence rate was 46.2% in 132 of 184 cases of α-FP < 100 ng/mL group. Of the 184 cases, α-FP 100–200 ng/mL were 14 cases (12 cases recurred (85.7%)). Alpha-fetoprotein > 200 ng/mL was 38 of 184 cases, 21 of which recurred (55.3%). According to the multivariate analysis, OR ratio was 8.003 (95% confidence interval [CI], 1.549–41.353) in the α-FP 100–200 ng/mL group and 1.867 (95% CI, 0.784–4.444) in α-FP 200 ng/mL or higher group (P = 0.013). Three-year survival rate of intrahepatic recurrence patients was 80.7%, 3-year survival rate of no intrahepatic recurrence patients was 95.0%.
Go to : 
Predicting hepatocellular carcinoma (HCC) recurrence is important for improving the prognosis of hepatectomy patients because of the rate of high recurrence after curative liver resection. Intrahepatic recurrence has a significant effect on the survival of HCC patients. The incidence of intrahepatic recurrence after liver resection is about 50% at 5 years for patients with solitary HCC [12].
The 5-year disease-free survival rate of HCC has been reported to be only 25%–30% [34]. Since frequent recurrence is the major obstacle for HCC treatment, many investigators have attempted to determine the important prognostic factors for HCC patients who undergo curative resection. Some of these factors include tumor size, tumor number, and portal vein invasion [5678].
Generally, tumor markers are used in the diagnosis, staging and prognosis of cancer and to monitor therapeutic effectiveness, or to detect recurrence. Alpha-fetoprotein, first introduced as a serological marker for HCC, has been commonly used to diagnose and monitor HCC [910]. However, it failed to be a reliable marker, revealing poor sensitivity, which necessitated a search for new markers for HCC [111213]. Des-γ-carboxy prothrombin (DCP), also known as a protein induced by vitamin K absence-II (PIVKA-II), is an abnormal prothrombin lacking carboxylation of 10 glutamic acid residues in the N-terminus that does not have coagulation activity [14]. Because α-FP and DCP are independently produced from HCC and show no correlation with each other, these 2 markers might serve as complementary markers for HCC [1516]. Therefore, combined measurement of these 2 markers appears to be useful in predicting the prognosis of HCC.
In healthy adults, the serum α-FP concentration is lower than 20 ng/mL. Alpha-fetoprotein plays an important role in regulation of oncogenic and ontogenetic growth [5]. Although an early study indicated that α-FP and its peptide fragments inhibit oncogenic growth, recent studies have shown that α-FP promotes HCC cell growth [7]. Alpha-fetoprotein-positive HCC has a higher cell proliferative rate than α-FP-negative HCC, and down regulation of α-FP suppresses HCC cell proliferation [7]. HBV-associated HCC is more tightly associated with a raised serum level of α-FP compared to HCV-associated HCC and non–virus-related liver cancer [14].
We investigated whether combined measurement of preoperative serum α-FP and PIVKA-II levels can predict recurrence after curative resection in HCC patients. We also examined whether the changes in tumor markers between preoperative and postoperative levels might influence the likelihood of HCC recurrence.
Go to : 
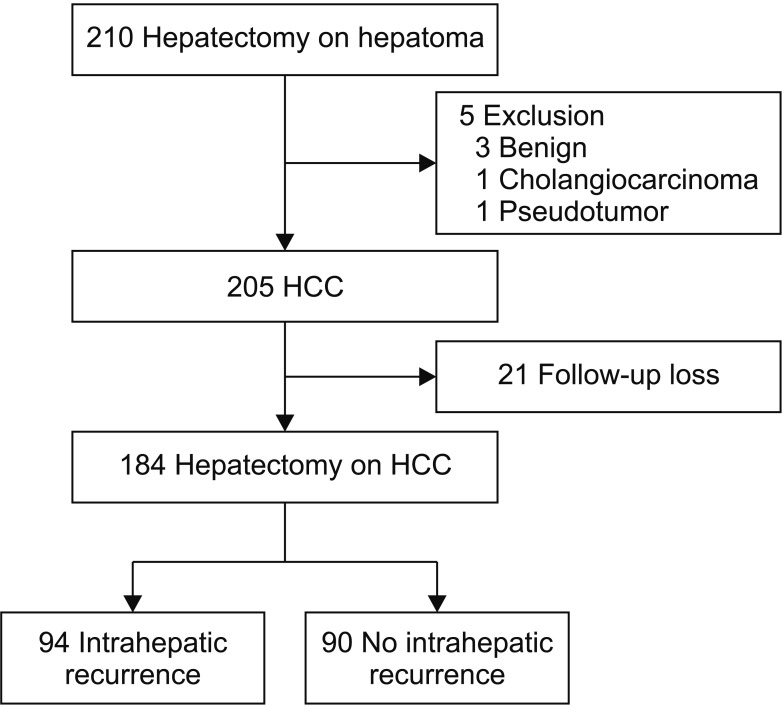
We retrospectively reviewed the medical records of 210 HCC patients who received hepatectomy between January 2009 and December 2015. Of the 210 patients, 184 patients were examined for α-FP and PIVKA-II serum levels, tumor burden, histologic grade, and other clinicopathological variables compared between the 2 groups of HCC patients as either intrahepatic recurrence or not (Fig. 1). This study was a retrospective study conducted in accordance with the Institutional Review Board approval from Pusan National University Hospital (PNUH 1801-027-063). Patients were not required to give informed consent to the study because the analysis used anonymous data that were obtained after each patient agreed to operation by written consent.
A-FP and DCP were measured at the time of preoperative examinations. Serum α-FP levels were determined by chemiluminescent immunoassays (Siemens Immulyze AFP IV; Mitsubishi Chemical Medience, Tokyo, Japan, and ARCHITECT A-FP EX; Abbott Japan, Tokyo, Japan). Serum DCP levels were determined by chemiluminescent enzyme immunoassay (Lumipulse PIVKA-II; Eisai, Tokyo, Japan).
Reference values of α-FP and DCP for detecting HCC in our institution were 12 ng/mL and 40 mAU/mL, respectively. In this study, tumor markers that were higher than these reference values were defined as positive markers for HCC.
Patients with well-preserved general function and liver function were candidates for surgery. All patients underwent hepatectomy based on hepatic vascular anatomy and the remnant liver volumes were estimated. Resection plans were determined by liver function and tumor location. If a tumor was located near the peripheral site, we performed wedge or partial resection, taking into account vascular anatomy.
Patients who survived surgery from 2009 to 2015 were followed up for at least 3 years. As of the second half of 2018, when the IRB documents were reviewed, data were continuously observed for patients who had been treated for the first time in 2009, and at least for 3 years for the patients treated in 2015.
All of the patients were followed up with blood examinations and ultrasonography or abdominal contrast-enhanced CT or MRI at our institute approximately every 3 months after operation. The image findings were reported by blinded radiologists and the presence of an intrahepatic recurrence was determined by the existence of a hypervascular nodule during the early phase with a perfusion detected in the portal phase under contrast-enhanced dynamic CT, MRI, or ultrasonography.
If an extrahepatic recurrence was suspected, then lung CT or bone scintigraphy was performed. When recurrence of HCC was detected, patients received further treatment for recurrent HCC, such as repeat hepatectomy, ablation therapy, or transarterial chemoembolization (TACE). After treatment for recurrent lesions, the same surveillance was performed.
When comparing clinical characteristics between intrahepatic recurrence group and no intrahepatic recurrence group, continuous variables were expressed as medians with interquartile ranges and compared using the Mann-Whitney U-test. Dichotomous variables were also compared using the chi-square test or Fisher exact test.
Three-year survival curves were calculated using the Kaplan-Meier method; the differences in survival and recurrence rates between intrahepatic recurrence group and no intrahepatic recurrence group were analyzed by the log-rank test. Multivariate Cox proportional hazard analyses were performed to identify independent prognostic risk factors for intrahepatic recurrence. All statistical analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC, USA) in this study. All P-values were 2-tailed and P < 0.05 was considered statistically significant.
Go to : 
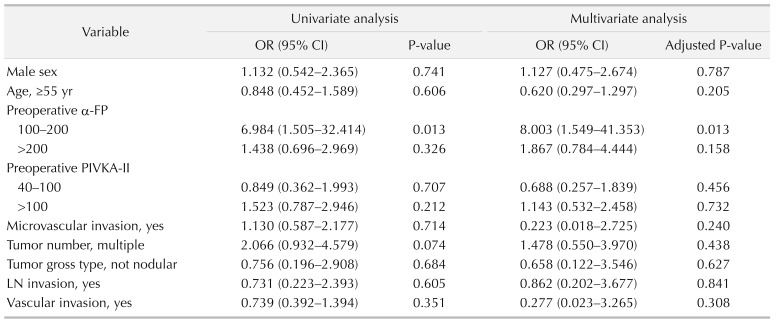
Of the 184 patients, we created 2 groups; 94 patients (51.1%) with intrahepatic recurrence and 90 patients (48.9%) without intrahepatic recurrence within the median follow-up period. For risk factors of intrahepatic recurrence, multivariate analysis showed operation type (hemihepatectomy), preoperative elevated α-FP, postoperative α-FP elevation, postoperative PIVKA-II elevation, and multiple tumor number were closely associated with intrahepatic recurrence (Table 1).
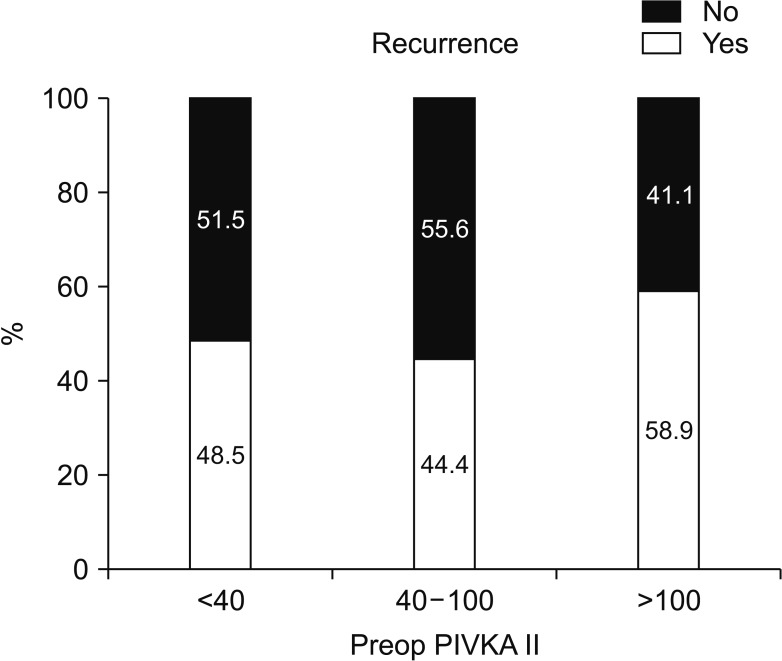
The factors affecting postoperative intrahepatic recurrence were analyzed according to preoperative DCP blood level (Fig. 2). In the group without postoperative intrahepatic recurrence, 52 of 90 patients (57.8%) had a preoperative PIVKA-II level of 40 IU or less. Preoperative PIVKA-II levels of 40–100 IU were; 15 of 90 patients (16.6%); over 100 IU were 23 of 90 patients (25.6%).
In the group with postoperative intrahepatic recurrence, 49 of 94 patients (52.1%) had a preoperative PIVKA-II level of 40 IU or less. Preoperative PIVKA-II levels of 40–100 IU were 12 of 94 patients (12.8%), over 100 IU, 33 of 94 patients (35.1%). The preoperative PIVKA-II (<40 IU, 40–100 IU, >100 IU) levels were not statistically significant in postoperative intrahepatic recurrence rate (Table 2).
The factors affecting postoperative intrahepatic recurrence were analyzed according to preoperative α-FP blood level (Fig. 3). In the group without postoperative intrahepatic recurrence, 71 of 90 patients (78.9%) had a preoperative α-FP II level of 100 ng/mL or less. Preoperative α-FP levels of 100–200 ng/mL were 2 of 90 patients (2.2%), over 200 ng/mL, 17 of 90 patients (18.9%). In the group with postoperative intrahepatic recurrence, 64 of 94 patients (68.1%) had a preoperative α-FP II level of 100 ng/mL or less. Preoperative α-FP levels of 100–200 ng/mL were 8 of 94 patients (8.5%), over 200 ng/mL, 49 of 94 patients (52.1%).
According to the multivariate analysis, odds ratio was 8.003 (95% confidence interval [CI], 1.549–41.353) in the α-FP 100–200 ng/mL group and 1.867 (95% CI, 0.784–4.444) in α-FP 200 ng/mL or higher group (P = 0.013) (Table 3). Given the total population of this study, it was concluded that the recurrence rate was high in preoperative α-FP > 100 ng/mL group. We attempted to determine the factors that affected the intrahepatic recurrence in patients who had postoperative recurrence by univariate and multivariate analysis (Tables 2, 3).
Postoperative α-FP levels (median range) were 4.22 ng/mL in the group without postoperative intrahepatic recurrence, 13.60 ng/mL in the group with postoperative intrahepatic recurrence.
Postoperative serum α-FP levels were not statistically significant for the effects of intrahepatic recurrence.
Lymph node invasion was not associated with intrahepatic recurrence. Three-year survival rate of intrahepatic recurrence patients was 80.7%, 3-year survival rate of no intrahepatic recurrence patients was 95.0%.
The factors that affected postoperative intrahepatic recurrence in the group in which the preoperative α-FP blood levels were analyzed are in Table 4.
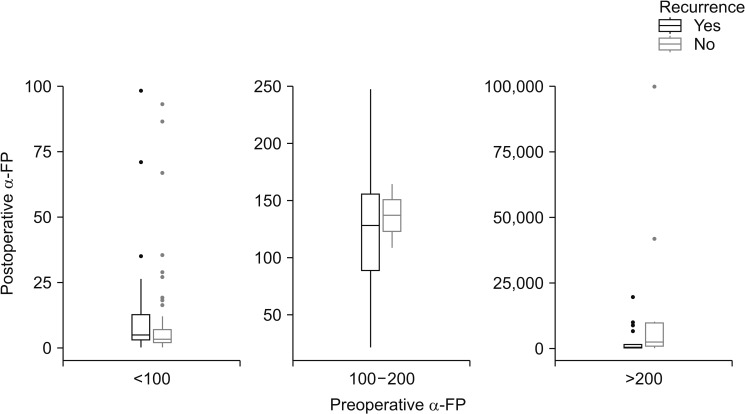
Also, we analyzed the postoperative recurrence relationship between preoperative and postoperative α-FP and PIVKA-II levels. There was no statistically significant difference (Figs. 4, 5).
Go to : 
In deciding how to treat a patient with high intrahepatic recurrence rates, many things could be considered. We wanted to find a significant factor in the recurrence rate of HCC after surgery with observing preoperative and postoperative changes of serum α-FP level, and analyzing the other factors that affect the recurrence rate of the liver. We evaluated tumor markers of HCC, namely α-FP, and DCP as indicators of intrahepatic recurrence and survival and predictors of patient outcome. The preoperative PIVKA-II level was not statistically significant in postoperative intrahepatic recurrence rate.
To study the association between serum α-FP and HCC prognosis, the different subtypes of HCC and varying underlying liver diseases should be taken into account more precisely. Another issue raised with respect to the use of serum α-FP as a prognostic marker is the optimal cutoff level. Previous studies have used different cutoff values ranging from 10 to 1,000 ng/mL [911151617].
The intrahepatic recurrence rate was low in the group with preoperative α-FP level of 100 ng/mL or less, whereas the relapse rate was high in the group with over 100 ng/mL. Meanwhile, multiple logistic regression analysis indicated that preoperative serum α-FP level (>200 ng/mL) was an independent prognostic factor for HCC postoperative survival rates. Results from our studies showed the α-FP level was an important prediction factor for the recurrence and prognosis of HCC after resection, and it could be used to evaluate the prognosis of HCC as an independent influential factor.
Postoperative α-FP level (median range) was 4.22 ng/mL in the group without postoperative intrahepatic recurrence, 13.60 ng/mL in the group with postoperative intrahepatic recurrence.
In many countries, including China, the incidence, recurrence, and mortality rates of liver cancer remain high. Clinical studies have shown that there is a close relationship between the level of serum α-FP and HCC incidence, recurrence and metastasis, and serum α-FP level has been used as the main index of prediction for HCC prognosis after hepatectomy [51011].
From pathological studies, there is literature showing that in normal liver tissue and serum α-FP-negative HCC, α-FP and α-FP receptors are not expressed; α-FP receptors are expressed only in the α-FP-positive HCC tissues [12]. Other scholars conducted a comparative study of the ultrastructural and immunohistochemical features of the α-FP-negative and -positive HCC tissues, and found the TN (Thomsen-Friedenreich-related antigen) protein expression level and positive rate in an α-FP-negative group was significantly higher than in an α-FP-positive group [13].
We also observed remarkable pathologically different features in intrahepatic recurrence group compared to no intrahepatic recurrence group; however, the recurrence rate and survival rate were not significantly different.
Rather, microvascular invasion has not been statistically shown as a significant factor of intrahepatic recurrence.
Our results showed that patients who had higher preoperative α-FP levels were more likely to experience recurrence on short-term follow-up. We need a longer follow-up period to confirm the long-term prognosis. This study was designed to report the response of relapse rate as preoperative α-FP level changes postoperatively. However, due to the small number of patients, many limitations appeared. Our paper examines the intrahepatic recurrences after liver resection for HCC in our hospital. After this paper is published, the next step is to compare intrahepatic recurrence with the treatment of radiofrequency ablation (RFA) and TACE according to the state of HCC. When it is done, the relationship between the factors that contributed to recurrence may be analyzed in more detail and the results may be known.
The OncoHepa test (CbsBioscience Inc., Daejeon, Korea), a multigene expression profile test developed by Shin Hwang, assesses the risk of tumor recurrence. Both OncoHepa test and α-FP-DCP-volume score (α-FP and DCP are multiplied by the tumor volume), have considerably strong prognostic power, and will be helpful in guiding postresection surveillance in patients with solitary HCCs ≤5 cm [18].
Consequently, analysis of factors carrying a high risk of recurrence may improve the selection of patients for surgery. Results from other studies have also shown α-FP level was an important prediction factor for the recurrence and prognosis of HCC after resection, TACE, or RFA treatment, and that it could be used to evaluate the prognosis of HCC as an independent influential factor [19202122].
In conclusion, the intrahepatic recurrence rate was low in the preoperative α-FP level of 100 ng/mL or less group, whereas the relapse rate was high in the over 100 ng/mL group. The preoperative PIVKA-II level was not statistically significant in postoperative intrahepatic recurrence rate.
Postoperative serum α-FP levels were not statistically significant for the effects of intrahepatic recurrence.
In high tumor markers, including not only preoperative and postoperative α-FP but also postoperative DCP, HCC patients who were received resection had early intrahepatic recurrence. Therefore, preoperative serum α-FP level has considerable predictive value for the malignant features and prognosis for patients with HCC.
On the other hand, for the HCC patients with postoperative α-FP elevation, long-term prognosis after resection needs to be further studied; our group will continue to follow up on these patients. Close follow-up is needed of patients with preoperative α-FP levels higher than 100 ng/mL. However, at the time of clinical diagnosis of liver cancer, the staging of HCC with preoperative high α-FP level was not different with preoperative low α-FP level, which may be related to the lack of extensive use of a highly sensitive screening index. The use of a sensitive serum biomarker to detect the early recurrence and metastasis of HCC is rare before signs are apparent on imaging studies. Novel serum markers that can predict recurrence in HCC with preoperative low α-FP level need further investigation. Prospective study for α-FP genes of HCC patients has been planned.
Based on the results of the current study, we revealed that the extent of malignancy and recurrence rate of α-FP-negative HCC is lower, survival rate is higher, and prognosis is better.
Go to : 
Notes
Fund/Grant Support: This work was supported by a 2-year Research Grant of Pusan National University.
Go to : 
References
1. Kim JM, Kwon CH, Joh JW, Park JB, Lee JH, Kim SJ, et al. Outcomes after curative hepatectomy in patients with non-B non-C hepatocellular carcinoma and hepatitis B virus hepatocellular carcinoma from non-cirrhotic liver. J Surg Oncol. 2014; 110:976–981. PMID: 25171344.

2. Kim JM, Kwon CH, Joh JW, Park JB, Lee JH, Kim SJ, et al. Differences between hepatocellular carcinoma and hepatitis B virus infection in patients with and without cirrhosis. Ann Surg Oncol. 2014; 21:458–465. PMID: 24132624.

3. Toyoda H, Kumada T, Tada T, Kaneoka Y, Maeda A. A laboratory marker, FIB-4 index, as a predictor for long-term outcomes of hepatocellular carcinoma patients after curative hepatic resection. Surgery. 2015; 157:699–707. PMID: 25704421.
4. Toyoda H, Kumada T, Tada T, Sone Y, Kaneoka Y, Maeda A. Tumor markers for hepatocellular carcinoma: simple and significant predictors of outcome in patients with HCC. Liver Cancer. 2015; 4:126–136. PMID: 26020034.

5. Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011; 53:1020–1022. PMID: 21374666.

6. Sato M, Tateishi R, Yasunaga H, Horiguchi H, Yoshida H, Matsuda S, et al. Mortality and morbidity of hepatectomy, radiofrequency ablation, and embolization for hepatocellular carcinoma: a national survey of 54,145 patients. J Gastroenterol. 2012; 47:1125–1133. PMID: 22426637.

7. Liu G, Ouyang Q, Xia F, Fan G, Yu J, Zhang C, et al. Alpha-fetoprotein response following transarterial chemoembolization indicates improved survival for intermediate-stage hepatocel lular carcinoma. HPB (Oxford). 2019; 21:107–113. PMID: 30017783.
8. Yang B, Zheng B, Yang M, Zeng Z, Yang F, Pu J, et al. Liver resection versus transarterial chemoembolization for the initial treatment of Barcelona Clinic Liver Cancer stage B hepatocellular carcinoma. Hepatol Int. 2018; 12:417–428. PMID: 30073454.

9. Ma WJ, Wang HY, Teng LS. Correlation analysis of preoperative serum alphafetoprotein (A-FP) level and prognosis of hepatocellular carcinoma (HCC) after hepatectomy. World J Surg Oncol. 2013; 11:212. PMID: 23981851.

10. Kamiyama T, Yokoo H, Kakisaka T, Orimo T, Wakayama K, Kamachi H, et al. Multiplication of alpha-fetoprotein and protein induced by vitamin K absence-II is a powerful predictor of prognosis and recurrence in hepatocellular carcinoma patients after a hepatectomy. Hepatol Res. 2015; 45:E21–E31. PMID: 25382703.

11. Chon YE, Choi GH, Lee MH, Kim SU, Kim DY, Ahn SH, et al. Combined measurement of preoperative α-fetoprotein and des-γ-carboxy prothrombin predicts recurrence after curative resection in patients with hepatitis-B-related hepatocellular carcinoma. Int J Cancer. 2012; 131:2332–2341. PMID: 22362471.

12. Kim SH, Moon DB, Kim WJ, Kang WH, Kwon JH, Jwa EK, et al. Preoperative prognostic values of α-fetoprotein (AFP) and protein induced by vitamin K absence or antagonist-II (PIVKA-II) in patients with hepatocellular carcinoma for living donor liver transplantation. Hepatobiliary Surg Nutr. 2016; 5:461–469. PMID: 28124000.

13. Ueno M, Hayami S, Shigekawa Y, Kawai M, Hirono S, Okada K, et al. Prognostic impact of surgery and radiofrequency ablation on single nodular HCC ≤5 cm: cohort study based on serum HCC markers. J Hepatol. 2015; 63:1352–1359. PMID: 26212030.

14. Tsugawa D, Fukumoto T, Kido M, Takebe A, Tanaka M, Kuramitsu K, et al. The predictive power of serum α-fetoprotein and des-γ-carboxy prothrombin for survival varies by tumor size in hepatocellular carcinoma. Kobe J Med Sci. 2015; 61:E124–E131. PMID: 27363395.
15. Meguro M, Mizuguchi T, Nishidate T, Okita K, Ishii M, Ota S, et al. Prognostic roles of preoperative α-fetoprotein and des-γ-carboxy prothrombin in hepatocellular carcinoma patients. World J Gastroenterol. 2015; 21:4933–4945. PMID: 25945007.

16. Parfitt JR, Marotta P, Alghamdi M, Wall W, Khakhar A, Suskin NG, et al. Recurrent hepatocellular carcinoma after transplantation: use of a pathological score on explanted livers to predict recurrence. Liver Transpl. 2007; 13:543–551. PMID: 17394152.

17. Lee MH, Kim SU, Kim DY, Ahn SH, Choi EH, Lee KH, et al. Early on-treatment predictions of clinical outcomes using alpha-fetoprotein and des-gamma-carboxy prothrombin responses in patients with advanced hepatocellular carcinoma. J Gastroenterol Hepatol. 2012; 27:313–322. PMID: 21793906.

18. Ha SM, Hwang S, Park JY, Lee YJ, Kim KH, Song GW, et al. Validation of the OncoHepa test, a multigene expression profile test, and the tumor marker-volume score to predict postresection outcome in small solitary hepatocellular carcinomas. Ann Surg Treat Res. 2018; 95:303–311. PMID: 30505821.

19. Li P, Wang SS, Liu H, Li N, McNutt MA, Li G, et al. Elevated serum alpha fetoprotein levels promote pathological progression of hepatocellular carcinoma. World J Gastroenterol. 2011; 17:4563–4571. PMID: 22147961.

20. Peng SY, Chen WJ, Lai PL, Jeng YM, Sheu JC, Hsu HC. High alpha-fetoprotein level correlates with high stage, early recurrence and poor prognosis of hepatocellular carcinoma: significance of hepatitis virus infection, age, p53 and beta-catenin mutations. Int J Cancer. 2004; 112:44–50. PMID: 15305374.
21. Yang SL, Liu LP, Yang S, Liu L, Ren JW, Fang X, et al. Preoperative serum α-fetoprotein and prognosis after hepatectomy for hepatocellular carcinoma. Br J Surg. 2016; 103:716–724. PMID: 26996727.

22. Torzilli G, Donadon M, Belghiti J, Kokudo N, Takayama T, Ferrero A, et al. Predicting individual survival after hepatectomy for hepatocellular carcinoma: a novel nomogram from the “HCC East & West Study Group”. J Gastrointest Surg. 2016; 20:1154–1162. PMID: 27003271.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download