Abstract
Purpose
The aim of this study was to determine the survival benefit based on different treatment strategies in patients with small, solitary, recurring intrahepatic hepatocellular carcinomas (HCCs) that were defined as recurred Barcelona Clinic Liver Cancer stage O (reBCLC-O).
Methods
Among the 917 patients with HCC recurrence after primary hepatic resection, 394 patients with reBCLC-O were selected. Of these, 150 patients underwent curative treatment (re-resection, radiofrequency ablation, and liver transplantation) and 203 underwent transarterial chemoembolization (TACE) group for recurrent HCC. After propensity score matching (PSM), both the groups were well balanced (89 patients in each group).
Results
Before PSM, the 1-, 3-, and 5-year overall survival (OS) rates of patients in the curative treatment group (96.7%, 78.6%, and 70.5%, respectively) were significantly better than those in the TACE treatment group (95.6%, 53.7%, and 44.2%, respectively) (P < 0.001). After PSM, the 1-, 3-, and 5-year OS rates also differed significantly (92.0%, 79.6%, and 71.1% in the curative treatment group vs. 88.8%, 65.6%, and 57.9% in the TACE group) (P = 0.005). The independent predictors of worse OS were tumor number at the time of resection and treatment modality for the recurrence, time interval to recurrence, and prothrombin time international normalized ratio and alpha-fetoprotein levels at the time of recurrence.
Go to : 
Hepatocellular carcinoma (HCC) is the second leading cause of cancer-related deaths worldwide [1]. The Barcelona Clinic Liver Cancer (BCLC) staging system has been widely accepted as a guideline for HCC treatment [2]. According to this system, liver resection, liver transplantation (LT), and local ablation are curative treatment modalities that improve survival outcomes in selected patients with good reserve liver function. Among these modalities, LT results in the best survival outcome. However, liver resection remains the first-line treatment option in many centers because of the severe organ shortage and increased waiting time. Over the past decade, the survival outcome of patients after liver resection has improved significantly because of advances in surgical skills and devices as well as postoperative management. However, long-term survival outcomes are unsatisfactory owing to the high recurrence rate after treatment [34567]. Most studies show a high incidence of intrahepatic HCC recurrence [89101112]. Moreover, to date, well-designed comparative studies on the treatment of intrahepatic HCC recurrence are sparse, and it is still unclear which treatment modality will guarantee better survival outcomes in patients with intrahepatic HCC recurrence.
Therefore, this study aimed to investigate the survival outcomes according to the treatment modality in selected patients with small (<2 cm), solitary, intrahepatic recurrent HCCs after primary liver resection that were classified as recurred Barcelona Clinic Liver Cancer stage O (reBCLC-O) and to determine the risk factors associated with survival outcomes in these patients.
Go to : 
This study included all the patients with HCC recurrence who underwent primary hepatic resection at the Seoul National University Hospital and Samsung Medical Center in Korea, between 2005 and 2011. To evaluate survival outcome according to the treatment modality, patients with intrahepatic reBCLC-O were divided into 2 groups; the curative treatment group (treated with re-resection, salvage LT, or radiofrequency ablation [RFA]) and the transarterial chemoembolization (TACE) group.
The reBCLC-O was defined as a small (<2 cm), solitary HCC with a performance status score of 0–1 and Child-Pugh score A or B, regardless of the primary HCC stage and time interval to recurrence.
After primary hepatic resection, all patients were followed up every 3–4 months to check for recurrence by monitoring α-FP levels and using dynamic CT or MRI. Hepatic recurrence was defined as new lesions observed with at least one imaging modality according to the guidelines provided by the European Association for Study of the Liver and Korean Association for Study of the Liver [1314].
Statistical analysis was performed using IBM SPSS Statistics ver. 20 (IBM Corp., Armonk, NY, USA). Categorical data were compared using the Pearson chi-square test or Fisher exact test, as appropriate. Continuous data were compared using the Wilcoxon rank-sum test. A P-value of <0.05 was considered to indicate statistical significance. The overall survival (OS) rate was defined as the interval between the time of recurrence and patient's death. Survival curves were generated using the Kaplan-Meier method and compared using the log-rank test. To determine the risk factors for OS in the entire cohort, a stratified Cox proportional hazard regression model was used.
The propensity score matching (PSM) method was performed for 1:1 matching of the primarily selected 353 patients. The 2 matched groups (i.e., the curative resection group and the TACE group) were compared to examine co-variable balance and determine any significant differences in baseline co-variables. Propensity score values were generated for characteristics at baseline (age, sex, tumor size, and albumin level) and the time of recurrence (time interval to recurrence and α-FP level).
This study was performed in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Review Boards of Samsung Medical Center (2013-05-013) and Seoul National University Hospital (H1303-061-474). This study does not require patient consent.
Go to : 
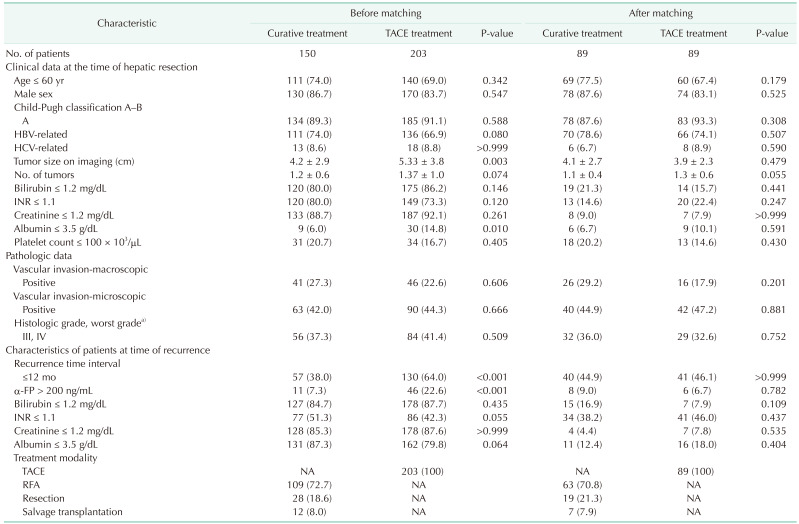
Among 917 patients with HCC recurrence after primary hepatic resection, 394 patients with reBCLC-O were selected (Fig. 1). Untreated patients (n = 9), patients who received percutaneous ethanol injection (n = 14), and patients who had positive pathologic margins (n = 18) were excluded. No patient underwent systemic therapy for reBCLC-O. Among the 353 included patients, 150 (42.5%) were in the curative treatment group and 203 (57.5%) in the TACE group. Several characteristics of the patients were significantly different between the 2 groups (Table 1). In the curative treatment group, we observed smaller tumors, lower baseline albumin levels, patients with a time interval of ≤12 months until tumor recurrence, and lower α-FP levels at the time of recurrence (P < 0.05 for all).
After PSM, both groups were well matched (89 patients in each group); the patient characteristics are summarized in Table 1. Of the 89 patients in the curative treatment group, 63 (70.8%) underwent RFA, 19 (21.3%) underwent re-resection, and 7 (7.9%) underwent salvage LT.
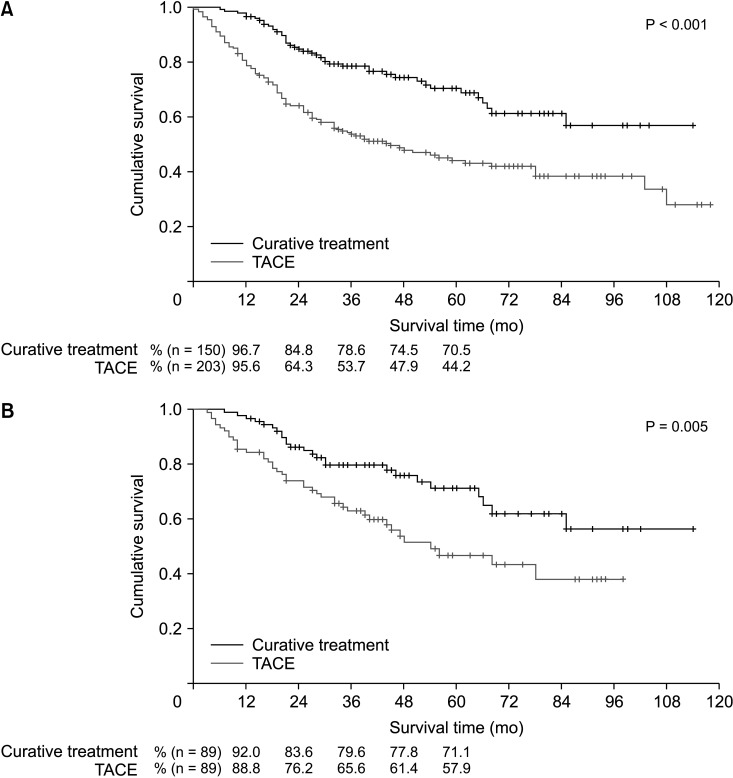
Before PSM, the patients in the curative treatment group exhibited a significantly better OS than those in the TACE treatment group (P < 0.001) (Fig. 2A). The estimated 1-year, 3-year, and 5-year OS rates were 96.7%, 78.6%, and 70.5%, respectively, for the curative treatment group and 95.6%, 53.7%, and 44.2%, respectively, for the TACE treatment group.
After PSM, there was a significant difference in the OS between the groups (P = 0.005) (Fig. 2B). The estimated 1-year, 3-year, and 5-year OS rates were 92.0%, 79.6%, and 71.1%, respectively, for the curative treatment group and 88.8%, 65.6%, and 57.9%, respectively, for the TACE treatment group.
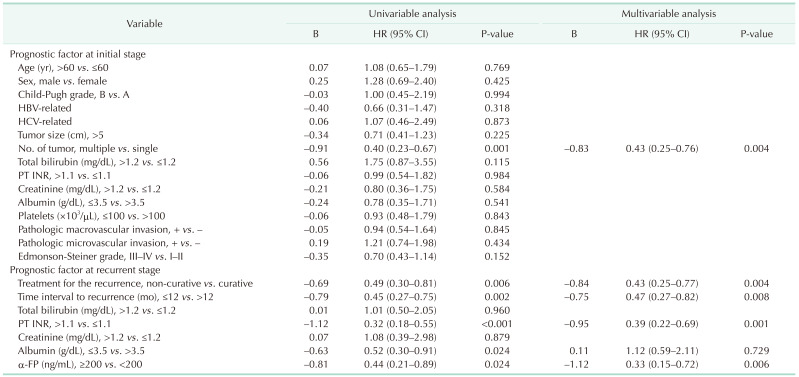
After PSM, the prognostic factors among the baseline characteristics were analyzed using a Cox proportional hazards model. Univariate analysis of baseline characteristics revealed that the primary prognostic factor for worse OS was the initial number of tumors (hazard ratio [HR], 0.40; 95% confidence interval [CI], 0.23–0.67; P = 0.001). Univariate analysis of the factors at the time of recurrence revealed that the prognostic factors for worse OS were treatment for recurrence (HR, 0.49; 95% CI, 0.30–0.81; P = 0.006), time interval to recurrence (HR, 0.45; 95% CI, 0.27–0.75; P = 0.002), INR level (HR, 0.32; 95% CI, 0.18–0.55; P < 0.001), albumin level (HR, 4.95; 95% CI, 1.82–13.46; P = 0.002), and α-FP level (HR, 0.44; 95% CI, 0.21–0.89; P = 0.024; Table 2).
Multivariate analysis revealed that the independent risk factors for worse OS were the initial number of tumors (HR, 0.43; 95% CI, 0.25–0.76; P = 0.004) among the baseline characteristics and treatment for the recurrence (HR, 0.43; 95% CI, 0.25–0.77; P = 0.004), time interval to recurrence (HR, 0.47; 95% CI, 0.27–0.82; P = 0.008), INR level at the time of recurrence (HR, 0.39; 95% CI, 0.22–0.69; P = 0.001) and α-FP level at the time of recurrence (HR, 0.33; 95% CI, 0.15–0.72; P = 0.006; Table 2).
Go to : 
In the present study, we demonstrated that curative treatment is the preferred option for patients with small (<2 cm), solitary, recurring intrahepatic HCC carcinoma that was defined as reBCLC-O. There were 2 major findings. One is that curative treatment did result in significantly better survival outcomes than TACE after patient selection using PSM. The second finding was that curative treatment was an independent prognostic factor in multivariate analysis.
Several curative treatments have been proposed for early-stage intrahepatic recurrent HCC over the past decades. Curative treatments consist of 3 treatment modalities: repeated hepatectomy, RFA, and salvage LT. Repeated hepatectomy has provided favorable outcomes in treating recurrent HCC; however, only a minority of patients are eligible for this because most patients have liver cirrhosis [151617]. Therefore, a strategy of primary resection and salvage LT for intrahepatic recurrent HCC has been suggested [1819]. This strategy showed the best long-term survival outcome among the curative treatments; the outcome was comparable to that of primary LT. In several studies, RFA has yielded a survival outcome comparable to that of repeated hepatectomy. This is considered more effective and safer than repeated resection in the aspects of lower severity; when compared to repeated hepatectomy, RFA shows a lower incidence of complication [202122].
The results of the studies mentioned above support our results. One of the limitations seen in these studies was that the indications for each treatment modality were not consistent. To overcome this issue, this study limited the size and number of recurrent tumors, which enabled us to define specific treatment targets. In addition, this study is unique in that the results were derived using PSM to overcome the limitation of the retrospective study design.
Our study demonstrated that a single tumor at an initial stage, longer time interval to recurrence (more than 12 months), normal INR value, and lower serum α-FP level (<200 ng/mL) were correlated with better patient survival.
The causes of tumor recurrence after hepatic resection may vary depending on the timing of the recurrence after initial treatment. In many studies, early recurrence means recurrence within 1 year after surgery caused by micrometastasis around the tumor at the time of surgery, and late recurrence means recurrence of de novo tumor in background cirrhosis [232425].
Among these, the number of tumors and recurrence time interval have been considered as the tumor characteristics that are strongly correlated with the survival outcome [192627]. It is noteworthy that the initial tumor characteristics have a significant impact on patient survival after recurrence. Several studies have reported that α-FP and INR levels are also closely related to the prognosis in the setting of primary resection. The α-FP has value both as a prognostic marker and predicting the response to therapy [28]. INR level is well known and widely used as a component of the model for end-stage liver disease score, which predicts survival expectancy in end-stage liver disease [29]. However, our study demonstrates that the α-FP and INR levels even at the time of recurrence have an impact on the survival outcome.
Therefore, initial tumor characteristics as well as the liver function and α-FP level at the time of recurrence should be considered as impact factors that influence the outcome of treatment in the reBCLC-O patient group.
This study has some limitations. A selection bias was present as the study had a retrospective design. The choice of treatment modality might have been influenced by patient characteristics and preferences owing to the lack of guidelines for treatment of HCC recurrence. Thus, the patients in the 2 groups exhibited some differences in clinicopathological characteristics, which may have acted as confounders that affected the oncologic outcome. Nevertheless, the results of our study are significant because several confounding factors were corrected for using PSM. Therefore, the results of this study could help in deciding a suitable treatment modality for patients with recurrent HCC.
In conclusion, the OS of patients in the curative treatments group was better than that of patients in the TACE treatment group after PSM. Curative treatments for recurred HCC, the nature of the original HCC (number and time interval to recurrence), and the INR and α-FP levels at the time of recurrence were found to be important prognostic factors for patients with reBCLC-O. Therefore, based on our results, considering these prognostic factors, curative treatment is strongly recommended in the patients with reBCLC-O recurrence for better survival. Nevertheless, further prospective randomized studies are warranted to confirm these results.
Go to : 
Notes
Fund/Grant Support: This study was supported by a grant (BCRI19011, 2018-00075) of Biomedical Research Institute, Chonnam National University Hospital.
Go to : 
References
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–E386. PMID: 25220842.

2. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018; 68:723–750. PMID: 29624699.

3. Pawlik TM, Poon RT, Abdalla EK, Zorzi D, Ikai I, Curley SA, et al. Critical appraisal of the clinical and pathologic predictors of survival after resection of large hepatocellular carcinoma. Arch Surg. 2005; 140:450–457. PMID: 15897440.

4. Poon RT, Fan ST. Hepatectomy for hepatocellular carcinoma: patient selection and postoperative outcome. Liver Transpl. 2004; 10(2 Suppl 1):S39–S45.

5. Bismuth H, Chiche L, Adam R, Castaing D, Diamond T, Dennison A. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg. 1993; 218:145–151. PMID: 8393649.

6. Cha C, Fong Y, Jarnagin WR, Blumgart LH, DeMatteo RP. Predictors and patterns of recurrence after resection of hepatocellular carcinoma. J Am Coll Surg. 2003; 197:753–758. PMID: 14585409.

7. Cha CH, Ruo L, Fong Y, Jarnagin WR, Shia J, Blumgart LH, et al. Resection of hepatocellular carcinoma in patients otherwise eligible for transplantation. Ann Surg. 2003; 238:315–321. PMID: 14501497.

8. Lu X, Zhao H, Yang H, Mao Y, Sang X, Miao R, et al. A prospective clinical study on early recurrence of hepatocellular carcinoma after hepatectomy. J Surg Oncol. 2009; 100:488–493. PMID: 19653238.

9. Rahbari NN, Mehrabi A, Mollberg NM, Müller SA, Koch M, Büchler MW, et al. Hepatocellular carcinoma: current management and perspectives for the future. Ann Surg. 2011; 253:453–469. PMID: 21263310.
10. Arnaoutakis DJ, Mavros MN, Shen F, Alexandrescu S, Firoozmand A, Popescu I, et al. Recurrence patterns and prognostic factors in patients with hepatocellular carcinoma in noncirrhotic liver: a multi-institutional analysis. Ann Surg Oncol. 2014; 21:147–154. PMID: 23959056.

11. Hirokawa F, Hayashi M, Asakuma M, Shimizu T, Inoue Y, Uchiyama K. Risk factors and patterns of early recurrence after curative hepatectomy for hepatocellular carcinoma. Surg Oncol. 2016; 25:24–29. PMID: 26979637.

12. Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000; 232:10–24. PMID: 10862190.

13. Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines for liver cirrhosis: ascites and related complications. Clin Mol Hepatol. 2018; 24:230–277. PMID: 29991196.
14. European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018; 69:182–236. PMID: 29628281.
15. Arima K, Nitta H, Beppu T, Nakagawa S, Okabe H, Imai K, et al. Impact of repeated hepatectomy on liver regeneration in hepatocellular carcinoma: a propensity score-based analysis. Anticancer Res. 2019; 39:965–970. PMID: 30711982.

16. Ochiai T, Inoue H, Watanabe N, Ito H, Toma A, Morimura R, et al. Outcome of a second hepatectomy in octogenarians with hepatocellular carcinoma recurrence: single centre's experience. ANZ J Surg. 2019; 89:1270–1274. PMID: 31280497.

17. Ho CM, Lee PH, Shau WY, Ho MC, Wu YM, Hu RH. Survival in patients with recurrent hepatocellular carcinoma after primary hepatectomy: comparative effectiveness of treatment modalities. Surgery. 2012; 151:700–709. PMID: 22284764.

18. Ferrer-Fàbrega J, Forner A, Liccioni A, Miquel R, Molina V, Navasa M, et al. Prospective validation of ab initio liver transplantation in hepatocellular carcinoma upon detection of risk factors for recurrence after resection. Hepatology. 2016; 63:839–849. PMID: 26567038.
19. Zhang X, Li C, Wen T, Peng W, Yan L, Yang J. Treatment for intrahepatic recurrence after curative resection of hepatocellular carcinoma: salvage liver transplantation or re-resection/radiofrequency ablation? A Retrospective Cohort Study. Int J Surg. 2017; 46:178–185. PMID: 28890407.

20. Xia Y, Li J, Liu G, Wang K, Qian G, Lu Z, et al. Long-term effects of repeat hepatectomy vs percut aneous radiof requency ablation among patients with recurrent hepatocellular carcinoma: a randomized clinical trial. JAMA Oncol. 2020; 6:255–263. PMID: 31774468.
21. Feng Y, Wu H, Huang DQ, Xu C, Zheng H, Maeda M, et al. Radiofrequency ablation versus repeat resection for recurrent hepatocellular carcinoma (≤ 5 cm) after initial curative resection. Eur Radiol. 2020; 30:6357–6368. PMID: 32529568.

22. Lu LH, Mei J, Kan A, Ling YH, Li SH, Wei W, et al. Treatment optimization for recurrent hepatocellular carcinoma: repeat hepatic resection versus radiofrequency ablation. Cancer Med. 2020; 9:2997–3005. PMID: 32108433.

23. Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg. 1999; 229:216–222. PMID: 10024103.
24. Shimada M, Takenaka K, Gion T, Fujiwara Y, Kajiyama K, Maeda T, et al. Prognosis of recurrent hepatocellular carcinoma: a 10-year surgical experience in Japan. Gastroenterology. 1996; 111:720–726. PMID: 8780578.

25. Shah SA, Greig PD, Gallinger S, Cattral MS, Dixon E, Kim RD, et al. Factors associated with early recurrence after resection for hepatocellular carcinoma and outcomes. J Am Coll Surg. 2006; 202:275–283. PMID: 16427553.

26. Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016; 150:835–853. PMID: 26795574.

27. Shimoda M, Tago K, Shiraki T, Mori S, Kato M, Aoki T, et al. Risk factors for early recurrence of single lesion hepatocellular carcinoma after curative resection. World J Surg. 2016; 40:2466–2471. PMID: 27138886.

28. Wang Y, Chen Y, Ge N, Zhang L, Xie X, Zhang J, et al. Prognostic significance of alpha-fetoprotein status in the outcome of hepatocellular carcinoma after treatment of transarterial chemoembolization. Ann Surg Oncol. 2012; 19:3540–3546. PMID: 22532305.

29. Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003; 124:91–96. PMID: 12512033.

Go to : 




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download