Abstract
Purpose
This study was performed to compare the oncologic outcomes between nonradical management and total mesorectal excision in good responders after chemoradiotherapy.
Methods
We analyzed 75 patients, who underwent 14 watch-and-wait, 30 local excision, and 31 total mesorectal excision, in ycT0-1N0M0 based on magnetic resonance imaging after chemoradiotherapy for advanced mid-to-low rectal cancer in 3 referral hospitals. The nonradical management group underwent surveillance with additional sigmoidoscopy and rectal magnetic resonance imaging every 3–6 months within the first 2 years.
Results
Nonradical management group had more low-lying tumors (P < 0.001) and less lymph node metastasis based on magnetic resonance imaging (P = 0.004). However, cT stage, ycT, and ycN stage were not different between the 2 groups. With a median follow-up period of 64.7 months, the 5-year locoregional failure rate was higher in the nonradical management group than in the total mesorectal excision group (16.7% vs. 0%, P = 0.013). However, the 5-year overall survival and disease-free survival rates of the nonradical management and total mesorectal excision groups were not different (95.2% vs. 93.5%, P = 0.467; 76.4% vs. 83.6%, P = 0.665; respectively).
Total mesorectal excision (TME) after preoperative chemoradiotherapy (PCRT) remains the mainstay treatment strategy for locally advanced rectal cancer [123]. Although TME has shown oncologic safety in patients, concerns remain regarding its probable complications, such as perioperative morbidity, increased bowel, urologic and sexual dysfunctions, and permanent stoma formation [45]. Nonradical management including watch-and-wait or local excision may improve patient quality of life while preserving the oncologic outcomes [56789].
Nonradical management can be considered for patients who show good response to PCRT with minimal chance of lymph node metastasis as this strategy omits regional lymph node dissection [8101112]. Studies have shown high correlation between postchemoradiotherapy pathologic tumor stage (ypT) status and lymph node metastasis [13]; patients with ypT0-1 disease have significantly lower lymph node metastasis and better oncologic survival than ypT2 or higher disease, indicating that ypT0-1N0 patients are appropriate candidates for nonradical management [1314]. However, previous studies on nonradical management have focused on either local excision or the watch-and-wait strategy, and the inclusion criteria of these studies are inconsistent. Thus, there is limited scope for drawing clear oncologic conclusions about nonradical management [81015]. While no diagnostic modality can definitely predict ypT0-1N0 after PCRT for rectal cancer, MRI has been reported as a reliable tool for evaluating tumor response after PCRT [16]. This study aimed to compare the oncologic outcomes between nonradical management and TME in postchemoradiotherapy clinical stage (yc) T0-1N0 based on MRI after PCRT for mid-to-low rectal cancer.
This was a multicenter, retrospective, cross-sectional study performed in 3 referral hospitals. We included patients who underwent nonradical management in Seoul National University Hospital, National Cancer Center, and Seoul National University Bundang Hospital, and patients who received TME in Seoul National University Bundang Hospital between March 2004 and April 2018. The inclusion criteria were as follows: (1) cT2-3N0-2 rectal cancer before PCRT; (2) mid-to-low rectum location (anal verge ≤ 10 cm); (3) ycT0-1N0 on MRI after PCRT; and (4) no evidence of distant metastasis, history of malignancy, or coexisting malignancies at diagnosis. This study was approved by the Institutional Review Board of the Seoul University Hospital, National Cancer Center, and Seoul National University Bundang Hospital (No. B-2004-606-101). Requirement for informed consent was waived due to the retrospective design of the study.
An initial evaluation of the rectal cancer was conducted before PCRT. Colonoscopy with a biopsy was performed to confirm the pathologic diagnosis. Rectal MRI was used to determine the depth of tumor invasion and lymph node evaluation. MRI scans were acquired using 1.5T Gyroscan Intera, 3T Achieva, or 3T Ingenia MR scanners (Philips Medical Systems, Best, Netherlands). Abdominopelvic and chest CT with or without PET were used to identify distant metastasis. The PCRT consisted of 5-fluorouracil or capecitabine-based chemotherapy and concurrent conventional fractionated radiotherapy, as in our previous study [17]; patients with T2 low rectal cancer scheduled to undergo sphincter preservation also underwent the same [12]. Tumor response was assessed 4–12 weeks after PCRT completion with similar protocol to the baseline work-up. The treatment strategy after PCRT was based on physical examination, sigmoidoscopy, CT, and MRI. The ycT stage was determined by the local extent of tumor signal intensity relative to bowel layers based on T2-weighted MRI. A subtle hypointense wall thickening was considered postradiotherapy fibrosis, and the absence of intermediate signal on T2-weighted MRI, with no residual tumor, including a scar or small ulcer on digital rectal examination or colonoscopy, was considered complete response (or ycT0N0 stage). Nonradical management, including watch-and-wait or local excision, was offered as alternative treatments to good responders who exhibited complete response or MRI stages less than or equal to ycT1N0, at the surgeon's discretion [18]. The decision for nonradical management and TME was made using a multidisciplinary team approach. Patients were sufficiently informed of the risks and benefits of each treatment and an informed shared decision was made, as in our previous study [19]. For poor responders who did not attain complete response or ycT1N0 stage on MRI, curative surgery was recommended if they were deemed fit to undergo radical surgery. Watch-and-wait patients i cluded all patients who did not undergo surgical treatment. Local excision included the excision of the full thickness of the mesorectum with an adequate safety margin using direct vision or minimally invasive transanal surgery with no tumor on the frozen biopsy. TME was performed with complete removal of the rectum and the surrounding mesorectum, including the pararectal lymph nodes. TME included low- and ultralow anterior resection, abdominoperineal resection, and intersphincteric resection, either by laparoscopic or open surgery. For poor responders, the choice between rectal preservation and extraction was at the surgeon's or the patient's discretion. Patients received adjuvant chemotherapy based on the presence of a tumor at the resection margin, high-risk factors of lymphovascular invasion after local excision, or pathologic stage and medical condition after TME. Toxicities due to treatment were reported according to the Common Terminology Criteria for Adverse Events (version 5.0) [20].
The surveillance protocol was described in our previous study [21]. All 3 hospitals had similar surveillance follow-up practices. For the first 2 years, the patients were followed up for every 3 to 6 months, and every 6 months thereafter. History-taking, digital rectal examination, and laboratory examination including carcinoembryonic antigen were performed at each visit. Abdominopelvic CT was performed every 6 months with or without chest CT. Colonoscopy was performed after 1 year of treatment and then every 2 years. The patients with nonradical management underwent additional sigmoidoscopy and rectal MRI assessment every 3 months in the first 2 years and every 6 months for the next 3 years.
Patient characteristics, including age, sex, tumor location, carcinoembryonic antigen level, pathologic differentiation, clinical stage before and after PCRT, and patterns of failure, were compared using Student t-test and chi-square test. The survival status of the patients was determined using the database provided by Statistics Korea (KOSTAT, mdis.kostat. go.kr). Overall survival was calculated from the beginning of the PCRT to the day of death from any cause. Events for disease-free survival included death from any cause, recurrence, distant metastasis, and second primary malignancy [22]. Locoregional failure was defined as recurrence or regrowth in the rectum, anastomotic site, or intrapelvic lymph nodes. The Kaplan-Meier method was used to estimate overall survival and disease-free survival. The log-rank test was used to compare the survival curves. A P-value of less than 0.05 was considered statistically significant. Statistical analyses were conducted using R software ver. 4.0.1 (R Foundation for Statistical Computing, Vienna, Austria).
Out of 625 patients with mid-to-low rectal cancer who received PCRT in Seoul National University Bundang Hospital, 55 patients (8.8%) reached ycT0-1N0 on MRI; 31 received TME, and 24 underwent nonradical management. Twenty patients who received nonradical management in Seoul National University Hospital and National Cancer Center were added to the nonradical management group. Among the 44 patients in the nonradical management group, 30 received local excision and 14 received watch-and-wait. Comparison of the characteristics of the TME group (n = 31) and nonradical management group (n = 44) are detailed in Table 1. Low rectal cancer (anal verge < 5 cm) was more common in the nonradical management group (84.1% vs. 38.7%, P < 0.001). The frequency of lymph node metastasis before PCRT was higher in the TME group (34.1% vs. 71.0%, P = 0.004). Age, sex, carcinoembryonic antigen level, cT stage, ycT stage, ycN stage, and pathologic differentiation were not statistically different between the 2 groups.
Tumor response assessment and operations were performed at a median of 39 days (interquartile range [IQR], 33–49 days) and 50 days (IQR, 46–59 days), respectively, after PCRT. The pathologic findings of local excision and TME patients are described in Table 1. One patient with ypT2 after local excision received adjuvant chemotherapy with 5-fluorouracil and has remained disease-free. One patient who underwent local excision with ypT3 after PCRT for rectal cancer 1 cm from the anal verge underwent abdominopelvic resection after 2 months due to severe anal incontinence. Of the 61 patients who underwent surgery, 39 patients (63.9%) received adjuvant chemotherapy; 14 of 30 (46.7%) after local excision and 25 of 31 (80.6%) after TME. The postoperative complication rates did not differ between local excision and TME (13.3% vs. 22.6%, P = 0.348) (Table 2). Two patients had complications and needed surgical interventions; 1 from the local excision group and 1 from the TME group. However, no deaths occurred because of these complications.
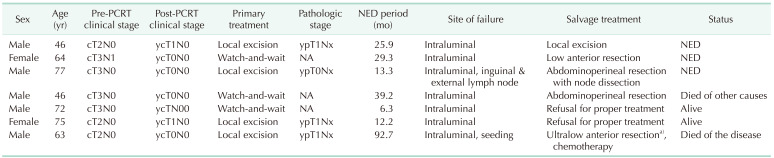
The patterns of treatment failure are summarized in Table 3. The nonradical management group had 7 locoregional failures, with or without concurrent distant metastasis, and 2 distant metastases. The TME group had one patient with distant metastasis. The total number of oncologic events were significantly different between groups (20.5% vs. 3.2%, P = 0.031). Table 4 summarizes the clinical information of the 7 patients who experienced locoregional failures. The median period with no evidence of disease was 25.9 months (range, 6.3–92.7 months). Four patients with locoregional failure were successfully treated with salvage surgery, but one of them died of other causes. Two patients refused proper treatment. One patient was found to have peritoneal seeding during the salvage operation and received cytoreductive surgery followed by adjuvant chemotherapy.
Comparisons of the Kaplan-Meier curves, with a median follow-up duration of 64.7 months (IQR, 34.5–94.7 months), are shown in Fig. 1. Differences between survival outcomes of the nonradical management and TME groups were not statistically significant; the estimated 5-year overall survival rates were 95.2% and 93.5%, respectively (P = 0.467), and the estimated 5-year disease-free survival rates were 76.4% and 83.6%, respectively (P = 0.665). The estimated 5-year locoregional failure rate was significantly higher in the nonradical management group than in the TME group (16.7% vs. 0%, P = 0.013). Five-year distant metastasis-free survival rates were not significantly different between the 2 groups (93.0% vs. 96.6%, P = 0.269) (Fig. 2). Oncologic outcomes within the nonradical management group were not significantly different between the watch-and-wait and local excision subgroups (Fig. 3).
This study showed that nonradical management including watch-and-wait or local excision in ycT0-1N0 based on MRI after PCRT for mid-to-low rectal cancer resulted in survival outcomes similar to those of standard radical surgery. Our results suggest that nonradical management might be an alternative treatment to TME for good responders after PCRT in advanced mid-to-low rectal cancer. In this study, we consider that salvage treatment under appropriate surveillance could manage locoregional recurrence or regrowth after nonradical management, despite the higher rate of locoregional events. However, further investigation is warranted to determine whether nonradical management is as safe as was seen in this study, because retrospective studies like the current one may have an unavoidable selection bias.
Our study compared the oncologic outcomes of nonradical management with that of TME for clinical good responders, especially ycT0-1N0 rectal cancer patients who can be managed by either watch-and-wait or local excision. Several previous studies compared local excision and TME based on good pathologic response [615], though they provided limited information on the guidance of nonradical management, as pathologic information can be obtained only after surgical intervention. In addition, some studies examined watch-and-wait and clinical complete responders to pathologic complete responders who underwent TME; however, they were unable to make an adequate comparison because of the low agreement in clinical and pathological stages [23]. Other studies have chosen different subject groups for the treatment comparisons: complete responders for watch-and-wait and non-complete responders for TME [824]. Few single institutional studies have used clinical good responders as subjects for both watch-and-wait and TME for oncologic comparison [25], which we have done in this study.
Although nonradical management may seem to be a heterogeneous group as it combines 2 strategies, our subgroup analysis showed that oncologic outcomes were not significantly different between the watch-and-wait and local excision patients. To the best of our knowledge, no study has compared watch-and-wait and local excision together with TME, except a meta-analysis study by Fiorica et al. [26]. The results of that study showed no significant survival difference between the nonradical management and TME groups as well as no difference in patients receiving watch-and-wait and local excision; this is consistent with our results.
The main concern on the clinical guidance of nonradical management is the need for a noninvasive tool for predicting pathologic response since many modalities have limitations in sensitivity [27]. Although MRI has low sensitivity, it remains one of the most reliable noninvasive modalities for evaluating PCRT response with an accuracy of 82% and specificity of 93% [1827]. Numerous studies support its ability to predict survival outcomes and its use in treatment selection after PCRT [161828]. Several studies also described encouraging results on the quality of MRI assessment with the progression of analysis protocols [29]. For these reasons, the 3 institutions primarily used MRI as the modality to select patients for nonradical management.
Oncologic safety can only be established when proper surveillance and timely salvage treatment are combined. With close surveillance protocols for the first 1 or 2 years, most studies have shown promising results for oncologic safety of nonradical management [7811]. A recent systematic review revealed that most of the locoregional failures occurred during the first 3 years of surveillance, with 2/3 occurring in the first year [7]. This suggests that surveillance protocols should include close observation, especially in the first 1 to 3 years. Our data showed no evidence of disease for a median period of 25.9 months before locoregional failure, and we performed surveillance similar to other studies of nonradical management with 3 to 6 months follow-up in the first 2 years [830]. Proper management of oncologic events in the nonradical management group was achieved with appropriate surveillance, which might explain the comparable overall survival rates in the nonradical management and TME despite the higher rates of oncologic events.
This study has some limitations. First of all, the non-randomized nature of the study over a long period was associated with unbalanced tumor locations, various clinical stages, and different chemotherapy treatments between the 2 groups, all of which influence oncologic outcomes. However, we consider that this was imposed by a gradual, but distinct, increase of cautious, nonradical management in the real-world clinical practice of rectal cancer. Second, the findings can be difficult to generalize because of the small sample size, as in other previous studies [22]. Due to the small sample size, a nearly 2-fold increase in the complication in the TME group showed no significant difference owing to insufficient statistical power.
In conclusion, this study showed that nonradical management for ycT0-1N0 mid-to-low rectal cancer may be an alternative treatment to TME when accompanied by proper surveillance and treatment for oncologic events. Our results confirm that management of oncologic events from nonradical management is possible with appropriate surveillance. In the future, prospective long-term studies with large subject numbers are necessary to support our conclusion on the oncologic safety of selecting patients for nonradical management based on MRI assessment.
References
1. Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012; 30:1926–1933. PMID: 22529255.

2. Uehara K, Nagino M. Neoadjuvant treatment for locally advanced rectal cancer: a systematic review. Surg Today. 2016; 46:161–168. PMID: 26170102.

3. Roh MS, Colangelo LH, O'Connell MJ, Yothers G, Deutsch M, Allegra CJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol. 2009; 27:5124–5130. PMID: 19770376.

4. Yoon WH, Kim HJ, Kim CH, Joo JK, Kim YJ, Kim HR. Oncologic impact of pathologic response on clinical outcome after preoperative chemoradiotherapy in locally advanced rectal cancer. Ann Surg Treat Res. 2015; 88:15–20. PMID: 25553320.

5. Pucciarelli S, Giandomenico F, De Paoli A, Gavaruzzi T, Lotto L, Mantello G, et al. Bowel function and quality of life after local excision or total mesorectal excision following chemoradiotherapy for rectal cancer. Br J Surg. 2017; 104:138–147. PMID: 27706805.

6. Hallam S, Messenger DE, Thomas MG. A systematic review of local excision after neoadjuvant therapy for rectal cancer: are ypt0 tumors the limit? Dis Colon Rectum. 2016; 59:984–997. PMID: 27602930.

7. Dattani M, Heald RJ, Goussous G, Broadhurst J, São Julião GP, Habr-Gama A, et al. Oncological and survival outcomes in watch and wait patients with a clinical complete response after neoadjuvant chemoradiotherapy for rectal cancer: a systematic review and pooled analysis. Ann Surg. 2018; 268:955–967. PMID: 29746338.
8. Renehan AG, Malcomson L, Emsley R, Gollins S, Maw A, Myint AS, et al. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol. 2016; 17:174–183. PMID: 26705854.

9. Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010; 11:835–844. PMID: 20692872.

10. Rullier E, Vendrely V, Asselineau J, Rouanet P, Tuech JJ, Valverde A, et al. Organ preservation with chemoradiotherapy plus local excision for rectal cancer: 5-year results of the GRECCAR 2 randomised trial. Lancet Gastroenterol Hepatol. 2020; 5:465–474. PMID: 32043980.

11. Shaikh I, Askari A, Ourû S, Warusavitarne J, Athanasiou T, Faiz O. Oncological outcomes of local excision compared with radical surgery after neoadjuvant chemoradiotherapy for rectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2015; 30:19–29. PMID: 25367179.

12. Garcia-Aguilar J, Renfro LA, Chow OS, Shi Q, Carrero XW, Lynn PB, et al. Organ preservation for clinical T2N0 distal rectal cancer using neoadjuvant chemoradiotherapy and local excision (ACOSOG Z6041): results of an open-label, single-arm, multi-institutional, phase 2 trial. Lancet Oncol. 2015; 16:1537–1546. PMID: 26474521.

13. Kim DW, Kim DY, Kim TH, Jung KH, Chang HJ, Sohn DK, et al. Is T classification still correlated with lymph node status after preoperative chemoradiotherapy for rectal cancer. Cancer. 2006; 106:1694–1700. PMID: 16532432.

14. Huang SH, Chi P, Lin HM, Lu XR, Huang YW, Xu ZB, et al. Selecting stage ypT0-1N0 for locally advanced rectal cancer following preoperative chemoradiotherapy: implications for potential candidates of organ-sparing management. Colorectal Dis. 2016; 18:989–996. PMID: 26880193.

15. Shin YS, Park JH, Yoon SM, Kim JC, Yu CS, Lim SB, et al. Total mesorectal excision versus local excision after preoperative chemoradiotherapy in rectal cancer with lymph node metastasis: a propensity score-matched analysis. Int J Radiat Oncol Biol Phys. 2018; 101:630–639. PMID: 29678529.
16. Balyasnikova S, Brown G. Optimal imaging strategies for rectal cancer staging and ongoing management. Curr Treat Options Oncol. 2016; 17:32. PMID: 27255100.

17. Jeong SY, Park JW, Nam BH, Kim S, Kang SB, Lim SB, et al. Open versus laparoscopic surgery for mid-rectal or low- rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol. 2014; 15:767–774. PMID: 24837215.
18. Patel UB, Taylor F, Blomqvist L, George C, Evans H, Tekkis P, et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol. 2011; 29:3753–3760. PMID: 21876084.

19. Kang SB, Cho JR, Jeong SY, Oh JH, Ahn S, Choi S, et al. Quality of life after sphincter preservation surgery or abdominoperineal resection for low rectal cancer (ASPIRE): a long-term prospective, multicentre, cohort study. Lancet Reg Health West Pac. 2021; 6:100087. PMID: 34327411.

20. National Cancer Institute (NCI). Common Terminology Criteria for Adverse Events (CTCAE). v5.0. Bethesda (MD): NCI;2017.
21. Song KS, Park SC, Sohn DK, Oh JH, Kim MJ, Park JW, et al. Oncologic risk of rectal preservation against medical advice after chemoradiotherapy for rectal cancer: a multicenter comparative cross-sectional study with rectal preservation as supported by surgeon. World J Surg. 2019; 43:3216–3223. PMID: 31410512.

22. Punt CJ, Buyse M, Köhne CH, Hohenberger P, Labianca R, Schmoll HJ, et al. Endpoints in adjuvant treatment trials: a systematic review of the literature in colon cancer and proposed definitions for future trials. J Natl Cancer Inst. 2007; 99:998–1003. PMID: 17596575.

23. Smith JD, Ruby JA, Goodman KA, Saltz LB, Guillem JG, Weiser MR, et al. Nonoperative management of rectal cancer with complete clinical response after neoadjuvant therapy. Ann Surg. 2012; 256:965–972. PMID: 23154394.

24. Appelt AL, Pløen J, Harling H, Jensen FS, Jensen LH, Jørgensen JC, et al. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol. 2015; 16:919–927. PMID: 26156652.

25. Lai CL, Lai MJ, Wu CC, Jao SW, Hsiao CW. Rectal cancer with complete clinical response after neoadjuvant chemoradiotherapy, surgery, or “watch and wait”. Int J Colorectal Dis. 2016; 31:413–419. PMID: 26607907.

26. Fiorica F, Trovò M, Anania G, Marcello D, Di Benedetto F, Marzola M, et al. Is it possible a conservative approach after radiochemotherapy in locally advanced rectal cancer (LARC)? A systematic review of the literature and meta-analysis. J Gastrointest Cancer. 2019; 50:98–108. PMID: 29273921.

27. Liu S, Zhong GX, Zhou WX, Xue HD, Pan WD, Xu L, et al. Can endorectal ultrasound, MRI, and mucosa integrity accurately predic t the complete response for mid-low rectal cancer after preoperative chemoradiation? A prospective observational study from a single medical center. Dis Colon Rectum. 2018; 61:903–910. PMID: 29944579.
28. Patel UB, Brown G, Rutten H, West N, Sebag-Montefiore D, Glynne-Jones R, et al. Comparison of magnetic resonance imaging and histopathological response to chemoradiotherapy i n local ly advanced rectal cancer. Ann Surg Oncol. 2012; 19:2842–2852. PMID: 22526897.
29. Nie K, Shi L, Chen ϱ, Hu X, Jabbour SK, Yue N, et al. Rectal cancer: assessment of neoadjuvant chemoradiation outcome based on radiomics of multiparametric MRI. Clin Cancer Res. 2016; 22:5256–5264. PMID: 27185368.

30. Rullier E, Rouanet P, Tuech JJ, Valverde A, Lelong B, Rivoire M, et al. Organ preservation for rectal cancer (GRECCAR 2): a prospective, randomised, open-label, multicentre, phase 3 trial. Lancet. 2017; 390:469–479. PMID: 28601342.

Fig. 1
Comparisons of the Kaplan-Meier survival curves of the nonradical management (NRM) and total mesorectal excision (TME) groups. (A) Overall survival (P = 0.467). (B) Disease-free survival (P = 0.665).
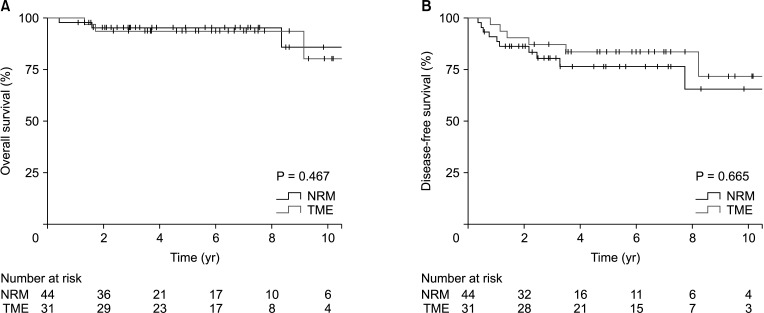
Fig. 2
Comparisons of the Kaplan-Meier survival curves of the nonradical management (NRM) and total mesorectal excision (TME) groups. (A) Locoregional failure-free survival (P = 0.013). (B) Distant metastasis-free survival (P = 0.269). *Statistically significant.
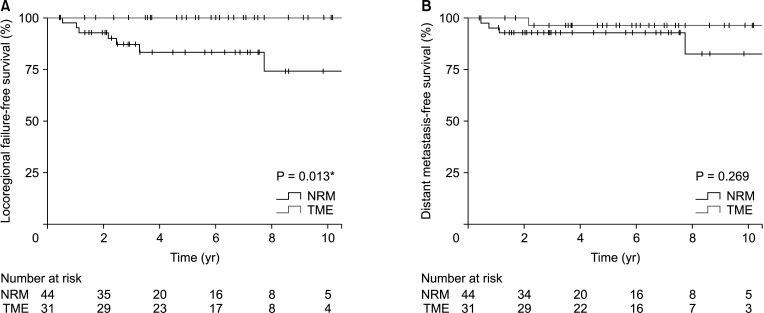
Fig. 3
Comparisons of the Kaplan-Meier survival curves of the watch-and-wait (WW) and local excision (LE) groups. (A) Overall survival (P = 0.866). (B) Disease-free survival (P = 0.818).
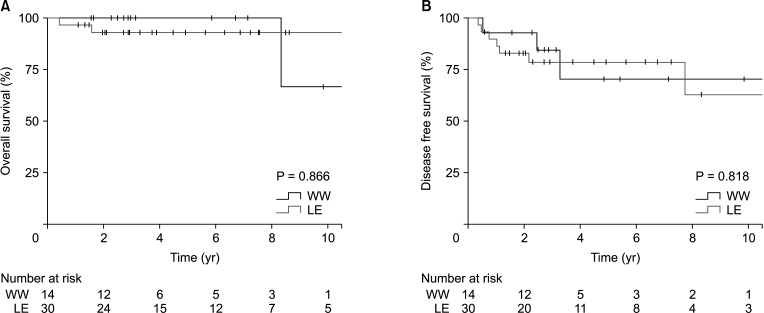
Table 1
Baseline characteristics of included patients
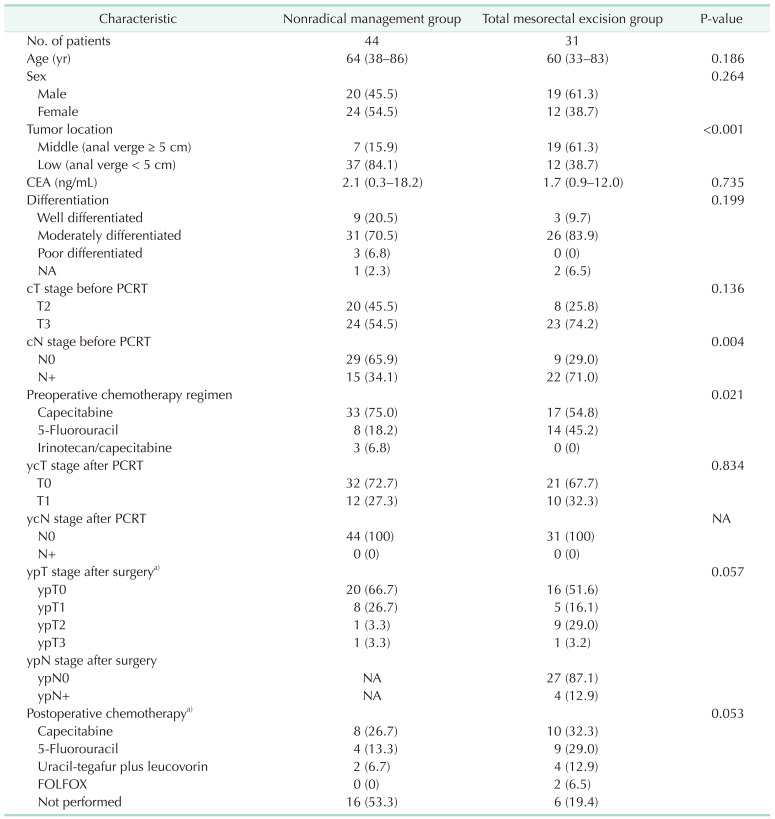
Values are presented as number only, number (%), or median (range).
PCRT, preoperative chemoradiotherapy; y, postchemoradiotherapy; c, clinical; p, pathologic; NA, not available; FOLFOX, folinic acid, fluorouracil, and oxaliplatin.
a)The local excision patients from nonradical management and total mesorectal excision patients were compared.




 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download