Abstract
Purpose
This study was performed to identify the risk of mortality in patients diagnosed with human epidermal growth factor receptor 2 (HER2)-positive ductal carcinoma in situ (DCIS).
Methods
We selected 2,592 patients with HER2-positive DCIS from Korean Breast Cancer Society (KBCS) database between January 1997 and December 2019. Patients who received neoadjuvant chemotherapy were excluded. Logistic regression analysis was used to determine the association between clinical factors and overall death after adjusting for tumor and clinical characteristics. Mortality data were modified using the Statistics Korea data.
Results
Thirty deaths (1.2%) were identified out of 2,592 patients in the KBCS database. In the univariate logistic regression analysis, older age, higher body mass index (BMI), type of breast surgery (mastectomy), estrogen receptor-negative, progesterone receptor-negative, and exposure to endocrine therapy were significant clinical factors associated with death. In the multivariate analysis, age (hazard ratio [HR], 1.062; 95% confidence interval [CI], 1.015–1.111; P = 0.006), BMI (HR, 1.179; 95% CI, 1.032–1.347, P = 0.016), breast surgery type (mastectomy vs. lumpectomy; HR, 0.285; 95% CI, 0.096–0.844; P = 0.024), and endocrine therapy (HR, 0.314; 95% CI, 0.099–0.995; P = 0.049) were significant risk factors for mortality.
Breast cancer is the most common cancer, and the second most common cause of cancer death among women in South Korea [1]. In 2017, approximately 22,400 new cases of invasive breast cancer occurred, and 2,500 patients died in Korea. Ductal carcinoma in situ (DCIS) accounts for approximately 15%–30% of newly diagnosed breast cancer [1]. DCIS is considered a precursor to invasive breast cancer [2], which explains the intense interest in molecular signaling pathways underlying breast cancer progression. Similar to invasive breast cancer, DCIS has different subtypes based on diverse molecular patterns [3]. Except for the use of tamoxifen in hormone receptor-positive DCIS, effective targeted therapies for other subtypes of DCIS have yet to be reported [4].
Overexpression of human epidermal growth factor receptor 2 (HER2) is associated with a high histological grade, p53 mutations, and absence of estrogen receptor (ER) [5]. In addition, the overexpression of HER2 is related to increased risk of breast cancer [6]. However, few studies have reported the clinical and survival data of patients with HER2-positive DCIS in Korea.
To evaluate the risk of mortality in patients with HER2-positive DCIS, we retrospectively analyzed the clinicopathological characteristics of patients in the Korean Breast Cancer Society Registry (KBCSR) database including 2 groups of patients (with or without death).
This retrospective cohort study used nationally representative data collected from the KBCSR database over a 23-year period from January 1997 to December 2019. KBCSR database includes electronic medical data such as age at surgery, weight, height, body mass index (BMI), date of diagnosis, date of surgery, the pathologic status of breast cancer (including DCIS), socioeconomic status, treatment modalities, and overall survival (OS) from nation-wide hospital-based registry. Survival results are updated annually with the support of the National Statistical Office in Korea (Statistics Korea). We analyzed data of patients who had been diagnosed with HER2-positive DCIS and underwent breast surgery. Exclusion criteria were: (1) diagnosis of invasive breast cancer, (2) unknown pathologic information including TNM stage, (3) history of neoadjuvant treatment, and (4) history of adjuvant chemotherapy.
In this study, all individual information was anonymized prior to data processing to comply with the privacy guidelines of the Health Insurance Portability and Accountability Act in Korea. The study protocol was approved by the Institutional Review Board of the Seoul St. Mary Hospital (No. KC21ZIDI0462), which waived the need for informed consent based on the retrospective cohort design. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki.
The main outcomes of interest were the differences of patients with and without survival in HER2-positive DCIS. In addition, we identified the clinical characteristics of HER2-positive DCIS. Continuous variables were compared between the 2 groups using the Student t-test or Mann-Whitney U-test. Categorical variables were compared using the chi-square test or Fisher exact test. Univariate and multivariate Cox regression models were used to identify factors associated with OS. The variables used in the multivariate Cox regression models were those that showed statistical significance in the univariate analysis. OS was defined as the period from primary curative surgery to the date of death due to any cause or the last follow-up. Data of patients who did not manifest an event of interest were censored at the date of the last follow-up.
IBM SPSS Statistics ver. 24 (IBM Corp., Armonk, NY, USA) was used for statistical analyses. Statistical significance was defined as a P-value less than 0.05, and the 95% confidence interval (CI) not including 1 was determined.
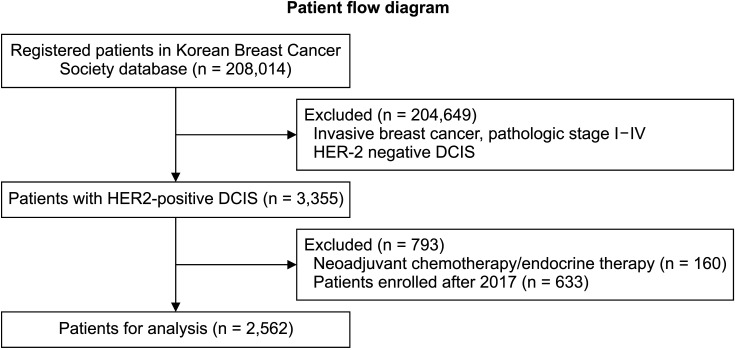
A total of 208,014 registered patients in Korean Breast Cancer Society database were analyzed in this study (Fig. 1). We included 3,355 patients with DCIS and HER2-positive. After excluding 793 patients who underwent neoadjuvant chemotherapy/endocrine therapy or enrolled after 2017, a total of 2,592 patients were included in this study. Among them, 30 patients (1.2%) died. Fifteen of them were identified with the death code (breast cancer, 8; lung cancer, 1; endometrial cancer, 1; extrahepatic bile duct cancer, 1; digestive tract cancer, 1; pneumonia, 1; tuberculosis, 1; and asphyxiation, 1). The median follow-up time was 72 months (range, 1–228 months).
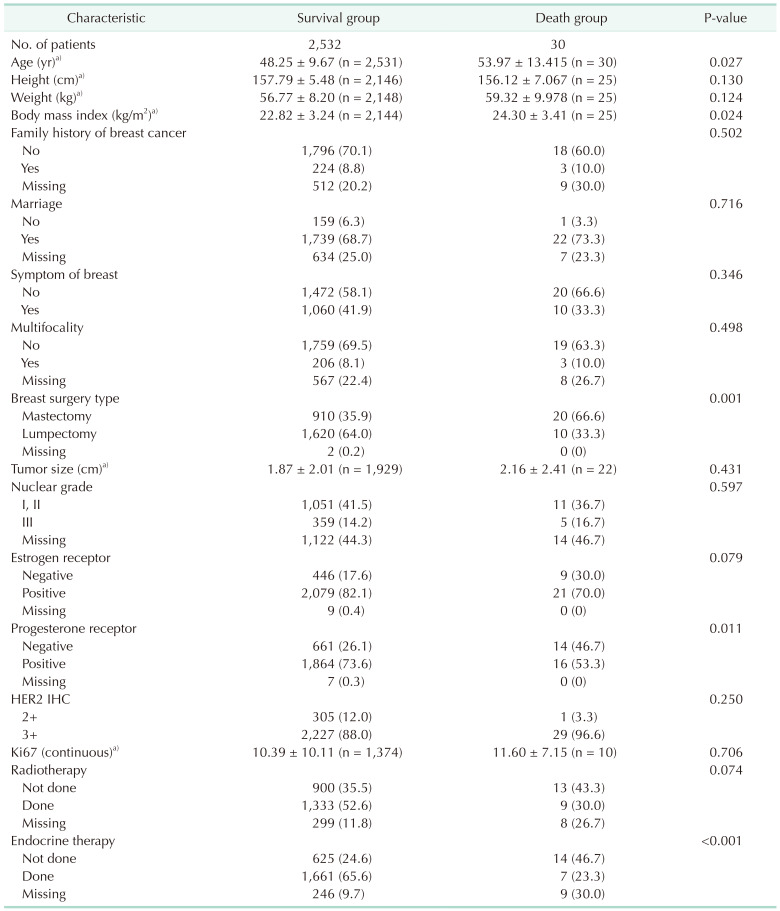
Clinical characteristics were grouped according to survival and compared based on survival status (Table 1). Compared with the dead patient, the alive patients were younger (48.25 ± 9.67 years vs. 53.97 ± 13.42 years, P = 0.027), with a lower BMI (22.82 ± 3.24 kg/m2
vs. 24.30 ± 3.41 kg/m2, P = 0.024), treated with breast surgery (mastectomy rate; 35.5% vs. 66.7%, P = 0.001), progesterone receptor (PR)-positive (72.8% vs. 53.3%, P = 0.011), and received tamoxifen treatment (64.8% vs. 23.3%, P < 0.001). No statistically significant differences were found in the size of DCIS, exposure to radiotherapy, immunohistochemical expression of HER2 (2+ vs. 3+), ER+ expression, nuclear grade, multifocality, presence of breast symptoms (palpability vs. non-palpability), age at surgery, and family history of breast cancer.
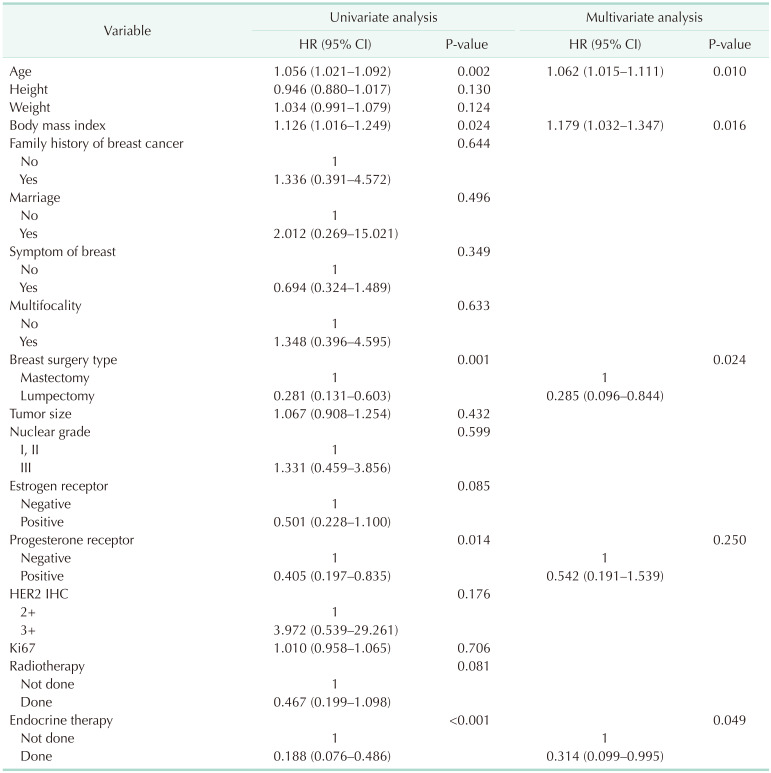
In univariate Cox regression analysis for OS, age at surgery (hazard ratio [HR], 1.056; 95% CI, 1.021–1.092; P = 0.002), BMI (HR, 1.126; 95% CI, 1.016–1.249; P = 0.024), positive PR expression (HR, 0.405; 95% CI, 0.197–0.835; P = 0.014), lumpectomy vs. mastectomy (HR, 0.281; 95% CI, 0.131–0.603; P = 0.001), and exposure to endocrine therapy (HR, 0.188; 95% CI, 0.076–0.486; P < 0.001) were significant factors (Table 2). After adjustment for the significant factors in univariate analysis, age at surgery (HR, 1.062; 95% CI, 1.015–1.111; P = 0.010), BMI (HR, 1.179; 95% CI, 1.032–1.347; P = 0.016), type of breast surgery (HR, 0.285; 95% CI, 0.096–0.844; P = 0.024), and exposure to endocrine therapy (HR, 0.314; 95% CI, 0.099–0.995; P = 0.049) were significant factors determining the OS (Table 2).
Clinical characteristics based on survival in the mastectomy group were compared in Supplementary Table 1. Survival group was associated with lower weight, BMI, and receipt of endocrine therapy, not ER and PR expression. In patients who underwent mastectomy with HER2 positive DCIS, age, weight, BMI, and receipt of endocrine therapy were significant factors in the univariate logistic regression analysis of survival (Supplementary Table 2). When adjusted for age, weight, BMI, and receipt of endocrine therapy was significant factor for survival (HR, 0.067; 95% CI, 0.008–0.543; P = 0.011) (Supplementary Table 2).
Clinical characteristics were compared in patients who underwent lumpectomy (Supplementary Table 3). Contrary to the results in the mastectomy group, the survival group showed taller and less PR negative in the lumpectomy group. Univariate analysis of survival in lumpectomy group was performed, height, age, PR expression, and multifocality were significant factors for survival (Supplementary Table 4). In the multivariate analysis, height and multifocality were significant factors for survival.
Historically, DCIS was treated by total mastectomy. Following the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-17 clinical trial [7], lumpectomy followed by radiotherapy was added as the standard treatment for selected patients. In NSABP B-24, the addition of tamoxifen further reduced the risk of recurrence, especially involving the ipsilateral breast, in patients with hormone receptor-positive DCIS [8]. The use of tamoxifen to treat hormone receptor-positive DCIS is the sole targeted treatment option for DCIS. Similar to invasive breast cancer, DCIS can be classified into various subtypes. However, unlike invasive breast cancer, currently no additional target therapy is available for the other subtype of DCIS. DCISs with high nuclear grade generally show negative ER and manifest HER2 overexpression frequently [91011]. Overexpression of HER2 in breast cancer is associated with high relapse of breast cancer and higher mortality [12].
In our study, the clinical characteristics of HER2-positive DCIS, survival outcome, and significant factors related to prolonged survival were identified. A total of 30 deaths occurred during a median follow-up of 72 months, resulting in a cumulative mortality rate of 1.2% in our study. Significant variables related to overall death in patients with HER2-positive DCIS were older age, high BMI, total mastectomy, and non-exposure to endocrine therapy.
In the pivotal trials NSABP B-17 and B-24 involving patients with DCIS, the cumulative incidence of all breast-cancer-related events (including any invasive disease and death) was 13% after radiotherapy in the B-17 and B-24 trials, and 8% after tamoxifen administration in B-24 [78]. The cumulative all-cause mortality over 15 years was 17.1% in patients exposed to adjuvant radiotherapy in the B-17 trial and 14.4% in the tamoxifen group of B-24 trial [13]. A total of 385 patients (14.7%) died in the treated group of B-17 and B-24 trials. Of the 385 deaths, 72 (18.7%) were attributed to breast cancer. In our study, the cumulative all-cause mortality was lower than in 2 pivotal studies probably due to the short follow-up period of this registry, younger age of enrolled patients, and the administration of adjuvant tamoxifen and radiotherapy.
Our findings suggest that the use of tamoxifen was a significant factor associated with survival, which was consistent with the results of NSABP B-24. The size of DCIS was not significantly related to survival, but total mastectomy was a poor prognostic factor associated with survival and the outcome may be affected by older age or mixed situation such as multifocality and/or larger tumor.
This study has some major limitations. First, there was no relapse data in the registry, and therefore it was not possible to analyze the factors increasing the risk of relapse in patients with HER2-positive DCIS. In addition, the total event of deaths was 30 cases, and the case itself was too small. Therefore, there were some limitations to statistical power. Second, although our sample size was large and included longitudinal data, the findings may not be generalizable to patients in other countries or patients of different ethnicities. Third, our study had a retrospective design based on analysis of the KBCSR registry. Finally, the median follow-up of 72 months was relatively shorter than in other studies.
Despite these limitations, our large population-based study demonstrated that factors such as older age, higher BMI, total mastectomy, and absence of endocrine therapy contributed to poor survival in patients with HER2-positive DCIS. A large-scale external study is needed to validate our findings.
References
1. Hong S, Won YJ, Park YR, Jung KW, Kong HJ, Lee ES, et al. Cancer Statistics in Korea: incidence, mortality, survival, and prevalence in 2017. Cancer Res Treat. 2020; 52:335–350. PMID: 32178489.

2. Page DL, Dupont WD, Rogers LW, Landenberger M. Intraductal carcinoma of the breast: follow-up after biopsy only. Cancer. 1982; 49:751–758. PMID: 6275978.

3. Bryan BB, Schnitt SJ, Collins LC. Ductal carcinoma in situ with basal-like phenotype: a possible precursor to invasive basal-like breast cancer. Mod Pathol. 2006; 19:617–621. PMID: 16528377.

4. Allred DC, Anderson SJ, Paik S, Wickerham DL, Nagtegaal ID, Swain SM, et al. Adjuvant tamoxifen reduces subsequent breast cancer in women with estrogen receptor-positive ductal carcinoma in situ: a study based on NSABP protocol B-24. J Clin Oncol. 2012; 30:1268–1273. PMID: 22393101.

5. Hoque A, Sneige N, Sahin AA, Menter DG, Bacus JW, Hortobagyi GN, et al. Her-2/neu gene amplification in ductal carcinoma in situ of the breast. Cancer Epidemiol Biomarkers Prev. 2002; 11:587–590. PMID: 12050101.
6. Curigliano G, Disalvatore D, Esposito A, Pruneri G, Lazzeroni M, Guerrieri-Gonzaga A, et al. Risk of subsequent in situ and invasive breast cancer in human epidermal growth factor receptor 2-positive ductal carcinoma in situ. Ann Oncol. 2015; 26:682–687. PMID: 25600567.
7. Fisher B, Dignam J, Wolmark N, Mamounas E, Costantino J, Poller W, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol. 1998; 16:441–452. PMID: 9469327.

8. Fisher B, Dignam J, Wolmark N, Wickerham DL, Fisher ER, Mamounas E, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999; 353:1993–2000. PMID: 10376613.

9. Claus EB, Chu P, Howe CL, Davison TL, Stern DF, Carter D, et al. Pathobiologic findings in DCIS of the breast: morphologic features, angiogenesis, HER-2/neu and hormone receptors. Exp Mol Pathol. 2001; 70:303–316. PMID: 11418009.

10. Collins LC, Schnitt SJ. HER2 protein over expression in estrogen receptor-positive ductal carcinoma in situ of the breast: frequency and implications for tamoxifen therapy. Mod Pathol. 2005; 18:615–620. PMID: 15696127.
11. Cornfield DB, Palazzo JP, Schwartz GF, Goonewardene SA, Kovatich AJ, Chervoneva I, et al. The prognostic significance of multiple morphologic features and biologic markers in ductal carcinoma in situ of the breast: a study of a large cohort of patients treated with surgery alone. Cancer. 2004; 100:2317–2327. PMID: 15160334.
12. Ross JS, Fletcher JA. The HER-2/neu oncogene in breast cancer: prognostic factor, predictive factor, and target for therapy. Stem Cells. 1998; 16:413–428. PMID: 9831867.
13. Wapnir IL, Dignam JJ, Fisher B, Mamounas EP, Anderson SJ, Julian TB, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst. 2011; 103:478–488. PMID: 21398619.

SUPPLEMENTARY MATERIALS
Supplementary Tables 1–4 can be found via https://doi.org/10.4174/astr.2021.101.6.315.
Supplementary Table 1
Clinical characteristics related to the survival in patients underwent mastectomy with HER2-positive DCIS
Supplementary Table 2
Hazard ratios (HRs) and 95% confidence intervals (CIs) for overall survival in patients underwent mastectomy with HER2-positive DCIS
Supplementary Table 3
Clinical characteristics related to the survival in patients underwent lumpectomy with HER2-positive DCIS
Supplementary Table 4
Hazard ratios (HRs) and 95% confidence intervals (CIs) for overall survival in patients underwent lumpectomy with HER2-positive DCIS




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download