Abstract
Purpose
The incidence of choledochal cyst (CC) with protein plugs is between 15.5%–40.4%. However, studies on CCs with protein plugs in children are limited. We aimed to analyze the clinical features, surgical findings, and complications of pediatric CCs with and without protein plugs.
Methods
We retrospectively analyzed 390 patients who underwent surgery for CCs between January 1987 and September 2017. The patients were divided into 2 groups: groups A (CC with protein plugs) and B (CC without protein plugs). The presence of protein plugs was evaluated using preoperative images or identified during surgery.
Results
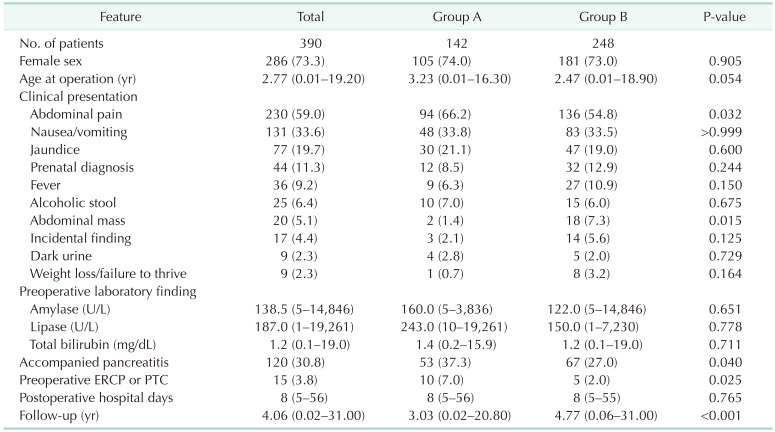
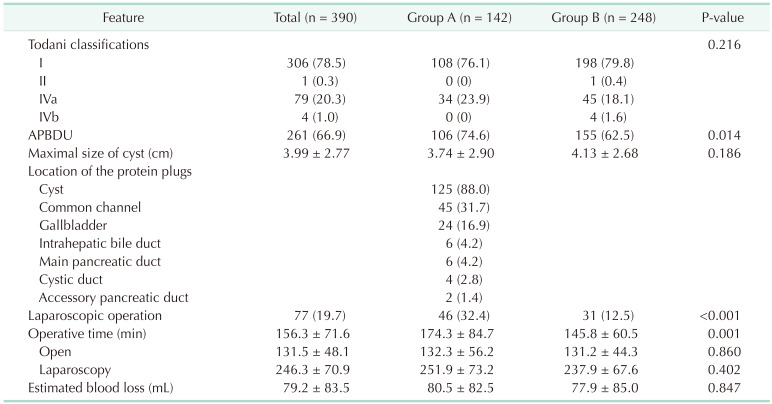
One hundred forty-two (36.4%) patients had protein plugs in the pancreaticobiliary duct. The most common initial clinical presentation was abdominal pain, and its incidence was significantly higher in group A (66.2%) than in group B (54.8%) (P = 0.032). The incidence of accompanying pancreatitis was also significantly higher in group A (37.3% vs. 27.0%) (P = 0.040). Anomalous pancreaticobiliary ductal union (APBDU) was found in 261 patients (66.9%) and its incidence was significantly higher in group A (74.6% vs. 62.5%) (P = 0.014). Most protein plugs were found in the cyst (88.0%) and common channel (31.7%). The incidence of early complications was higher in group A; conversely, that of late complications did not differ.
Choledochal cysts (CCs) are congenital anomalies involving cystic dilation of various intrahepatic or extrahepatic biliary trees [1]. They have a variety of symptoms in addition to the classic symptom triad (abdominal pain, jaundice, and palpable right upper quadrant mass). Owing to these symptoms, 80% of patients are diagnosed before the age of 10 years [2]. The incidence of CCs varies geographically, i.e., 1 in 100,000–150,000 live births in Western populations and 1 in 1,000 in Asian populations [3]. CCs are significantly more common in Asia. Additionally, the female to male ratio of incidence is approximately 3.5:1 [4].
In preoperative images or during surgery for CCs, sludge or stones are often found in the pancreaticobiliary ducts. Biliary sludge or stones increase in incidence as the patient ages and were found more frequently in Todani type II, IV, or V than in type I cysts [56]. Sludges or stones accompanied by CCs are radiolucent, and over 98% of the components are protein, which is called a protein plug [7]. These protein plugs were found in 15.5%–40% of patients with CCs, and their incidence is higher in children (41.2%) than in adults (12.5%) [789].
There are few studies on CCs with protein plugs in children. Although the characteristics of CCs with protein plugs in children have been reported previously, only a small number of patients were studied (11–38 participants) [56789]. These studies also did not clearly elucidate the incidence of CCs with protein plugs with age or the type of cyst [568]. Therefore, we aimed to compare the clinical features, surgical findings, and complications of CCs with and without protein plugs among children with CCs who underwent surgery at a single center.
Three hundred and ninety patients who underwent surgery for CCs in Seoul National University Children’s Hospital between January 1987 and September 2017 were retrospectively analyzed. The data collected included age at surgery, sex, initial clinical presentations, diagnostic tools, preoperative laboratory findings, intraoperative findings, anomalous pancreaticobiliary ductal union (APBDU), accompanying pancreatitis, and postoperative complications. The inclusion criteria were as follows: (1) pediatric patients aged <19 years with a history of surgery for CCs; (2) well-preserved records on initial clinical presentations, laboratory findings, and surgical records; and (3) APBDU evaluated through operative cholangiography (OPC), magnetic resonance cholangiopancreatography (MRCP), or abdominal CT. Cyst excision and Roux-en-Y hepaticojejunostomy (HJ) were performed routinely. Since 2009, the laparoscopic approach was actively performed.
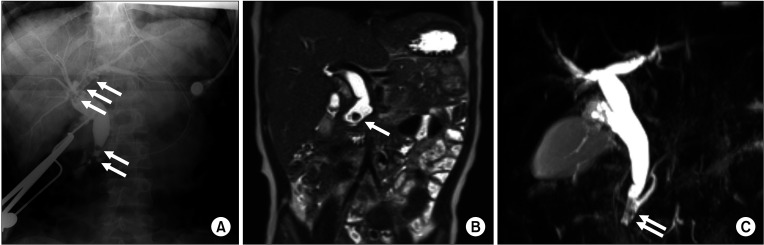
We divided the patients into 2 groups; groups A (CC with protein plugs) and B (CC without protein plugs). Protein plugs were identified when radiolucent filling defects of round or irregular shapes were noted in the pancreaticobiliary duct on preoperative imaging (Fig. 1).
APBDU was defined when the union between the common bile duct and pancreatic duct was located far from the duodenum, and the common channel length exceeded 5 mm [101112]. Herein, 389 patients were evaluated for APBDU via OPC (n = 309, 79.2%) or preoperative MRCP (n = 128, 32.8%) and 1 patient via abdominal CT.
Acute pancreatitis was defined as the presence of at least 2 of the following 3 criteria: (1) abdominal pain suggestive of or compatible with pancreatic origin, (2) serum amylase and/or lipase activity at least 3 times greater than the upper normal limit, and (3) imaging findings suggestive of or compatible with pancreatic inflammation [13].
Complications occurring within 30 days postoperatively were defined as early complications and those occurring after 30 days as late complications. At the postoperative follow-up, we performed annual ultrasonography to screen for potential complications.
Statistical comparisons were performed using Student t-test for continuous values and Pearson chi-square test for categorical values. Variables with median values were analyzed using the Mann-Whitney U-test. We performed logistic regression to calculate the odds ratios (ORs) and 95% confidence intervals. Statistical differences were considered significant at P-values of <0.05. Statistical analysis was performed using IBM SPSS Statistics ver. 20 (IBM Corp., Armonk, NY, USA).
This study was approved by the Institutional Review Board of Seoul National University Hospital (No. 1909-100-1066). The requirement for informed consent was waived because of the retrospective nature of the study.
Of the 390 patients, 142 (36.4%) had protein plugs in the pancreaticobiliary duct and were classified into group A. The proportion of female patients was 73.3% in group A and 73% in group B. The median age at surgery was 3.23 years (range, 0.01–16.3 years) in group A and 2.47 years (range, 0.01–18.9 years) in group B; however, the difference was not significant (P = 0.054). The most common initial clinical presentation was abdominal pain, and its incidence was significantly higher in group A (66.2%) than in group B (54.8%) (P = 0.032). Abdominal mass was found in 5.1% of patients, and the median age at surgery was 12.33 months (range, 0.47–92.33 months), and the median cystic size was 6.5 cm (range, 3–13 cm). The preoperative amylase, lipase, and total bilirubin levels were similar between the groups. The incidence of accompanying pancreatitis was also significantly higher in group A (37.3% vs. 27.0%) (P = 0.040). Preoperative biliary drainage was performed through endoscopic retrograde cholangiopancreatography (ERCP) or percutaneous transhepatic cholangiography (PTC) in 10 patients (7%) in group A and 5 patients (2%) in group B (P = 0.025) (Table 1).
The most common Todani type of CC was type I (78.5%), followed by type IVa (20.3%). The distribution of the CC types between the groups was similar. APBDU was found in 261 patients (66.9%), and its incidence was significantly higher in group A (74.6% vs. 62.5%) (P = 0.014). The protein plugs were most commonly found in the cyst (88.0%) and common channel (31.7%). In 60 patients, the protein plugs were found in several locations simultaneously. The operative time was significantly longer in group A, which could be because laparoscopic surgery was performed more often in this group (Table 2).
We analyzed and compared the significant risks in both groups. Patients with protein plugs had higher risks for abdominal pain (OR, 1.613), accompanying pancreatitis (OR, 1.609), and APBDU (OR, 1.767) than those without (Table 3).
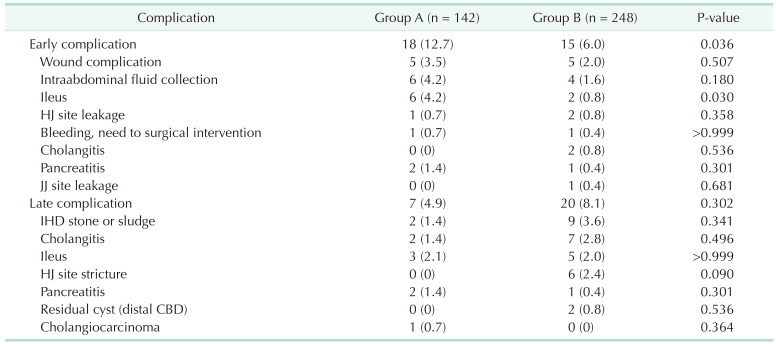
Early complications occurred in 33 patients (8.5%). The incidence of early complications was higher in group A (12.7% vs. 6.0%) (P = 0.036). Three patients had postoperative HJ site leakage, and 2 patients improved with conservative treatment. One patient underwent revision surgery owing to non-improvement even 30 days after surgery. Late complications occurred in 27 patients (6.9%). Intrahepatic duct stone or sludge occurred in 11 patients, and the incidence in groups A (1.4%) and B (3.6%) was statistically similar. In 8 of these patients, there was improvement after stone or sludge removal through percutaneous biliary intervention. Three patients improved after surgery. Among them, 2 had intrahepatic duct stones 11 and 21 years after CC excision, respectively, and HJ site revision was performed after stone removal through the HJ site. One patient underwent left hemihepatectomy 12 years after CC excision. Six patients (2.4%) had HJ site strictures in group B. Two patients improved after surgery and 4 after percutaneous biliary intervention. One patient who underwent surgery at the age of 13 years in group A with Todani type I was diagnosed with cholangiocarcinoma with multiple hepatic metastases after 21 years. We suggested patient treatment; however, the patient refused treatment, and subsequent follow-up failed (Table 4).
Many pediatric surgeons may have encountered protein plugs in the pancreaticobiliary duct when treating CCs. Why these protein plugs occur in CCs and how they affect clinical findings remain unclear. Therefore, we performed a retrospective review of patients with CCs who had undergone surgery in our hospital. Herein, 36.4% of the patients had protein plugs. In studies including adults, CC with protein plugs has been reported to occur in 15.5%–40.4% of patients [56789]. In these studies, when subjects were limited to pediatric age, the frequency of patients with protein plug was 15.5%–31.8% [5689]. Compared to previous studies, the high incidence of protein plugs detected in this study is probably due to the application of MRCP. Herein, 128 patients underwent MRCP, and 76 (59.4%) had protein plugs. Among those who did not undergo MRCP, 66 (25.2%) had protein plugs; this result was significantly different from that among those who did (P < 0.001). In fact, a study that analyzed pediatric CCs using MRCP reported that the incidence of protein plugs was 76.9% [14]. Therefore, the incidence of protein plugs in pediatric CCs is thought to be approximately ≥60%. In contrast, the incidence of protein plugs among those who did not undergo MRCP herein was low, which may be attributed to the spontaneous resolution of the protein plug. Symptoms, such as abdominal pain, nausea, vomiting, or jaundice, in CCs were claimed to be caused by disturbance of the biliary or pancreatic flow in common channels by protein plugs [715]. Protein plugs were very fragile and disappeared spontaneously or after flushing in 81.8% of cases. Further, 42.8% of protein plugs found before surgery were reported to have disappeared spontaneously during surgery [8]. Therefore, several protein plugs might be found during MRCP, which was performed when there were symptoms, such as abdominal pain. As most patients underwent surgery after these symptoms were resolved, the incidence of protein plugs might be low in OPC without MRCP.
Why protein plugs with CCs occur remains controversial. In one study, more than 98% of plug-or-stones were reported as protein [7]. Further, the major component of these proteins was lithostathine [15]. Lithostathine fibrils were found in the form of protein plugs or stones in the pancreatic duct of patients with chronic pancreatitis [1617]; it is only secreted in the pancreas, and its role remains unclear [18]. Because of this history, lithostathine is also called pancreatic stone protein, pancreatic thread protein, islet of Langerhans regenerating protein, or islet cell regenerating factor [19]. The most probable hypothesis underlying the presence of lithostathine in protein plugs in CCs is that the pancreatic juice containing lithostathine is refluxed to the biliary tract by APBDU, so that refluxed lithostathine makes lithostathine fibrils, which then become larger and are made into protein plugs [15]. Protein plugs can be distributed anywhere on the pancreaticobiliary duct, especially in narrowing areas, such as distal common bile ducts, common channels, or pancreatic ducts, which block the flow of bile or pancreatic juices and are thought to cause characteristic symptoms, such as abdominal pain, nausea/vomiting, jaundice, or pancreatitis.
The incidence of protein plugs or stones differs depending on the Todani type. Stones were reportedly found in 35% of type I cysts but in 71.4% of other cyst types [6]. Protein plugs were reportedly found in 14.3% of type I cysts and in 31.8% of type IVa cysts [8]. In this study, protein plugs were found in 35.3% of type I cysts and in 43.0% of type IVa cysts; however, the difference was not significant (P = 0.239). Other studies have reported different results for the incidence of protein plugs at different ages [568]. We analyzed the incidence by age and found that it was 31.2% at 0 years of age, 21.4% at 1 year, 40.3% at 2 years, 36.4% at 3 years, 44.1% at 4 years, 50% at 5 years, 46.7% at 6 years, 25.0% at 7 years, 44.4% at 8 years, 50.0% at 9 and 10 years, 66.7% at 11 years, 42.8% at 12 years, 66.7% at 13 years, 20.0% at 15 years, and 75.0% at 16 years of age. The incidence seemed to increase with age but the increase was not statistically significant (P = 0.065).
The incidence of protein plugs in the common channel was previously reported to be 8%–27.3% [7112021]. Herein, most protein plugs were found in the cysts (88.0%) and common channels (31.7%). Conversely, they were found in the intrahepatic bile duct, main pancreatic duct, and accessory pancreatic duct in 6, 6, and 2 patients, respectively. Therefore, we believe that common channel and intrahepatic duct irrigation are very important. In our institution, we dissect the distal common bile duct just within the head of the pancreas, then open the cyst, and perform transection after saline irrigation in the distal direction. In the upper part of the CC, HJ is performed after common hepatic duct transection at the bifurcation level, followed by careful saline intrahepatic duct irrigation.
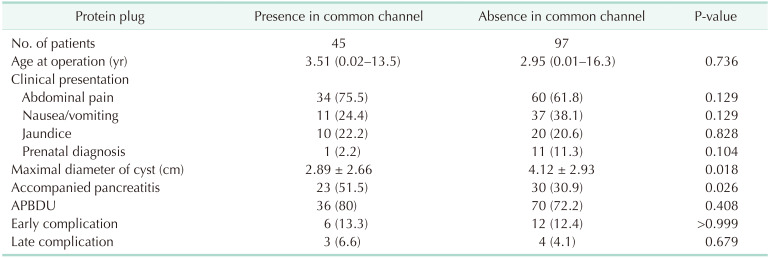
When protein plugs are present in the common channels, they cause more abdominal pain or pancreatitis [722]. We compared protein plugs found in the common channels to those found elsewhere in group A (Table 5). Abdominal pain occurred in 75.5% of those with protein plugs in the common channel and 61.8% of those without, but the difference was not significant (P = 0.129). In patients with protein plugs in the common channel, the maximal diameter of CC was smaller than those without (2.89 ± 2.66 cm vs. 4.12 ± 2.93 cm, P = 0.018). Of the 45 patients with protein plugs in the common channel, 23 (51.5%) had pancreatitis; conversely, of the 97 patients with protein plugs elsewhere, 30 (30.9%) had pancreatitis (P = 0.026). Pancreatitis was then more often accompanied by protein plugs in the common channel. APBDU was found in 80% of those with protein plugs in the common channel and 72.2% of those without; and the difference was not significant (P = 0.408). The other chief symptoms, surgical findings, or complications were not related to the protein plug location.
Herein, 15 patients underwent preoperative biliary drainage for persistent abdominal pain, fever, or jaundice. Nine of the 10 group A patients underwent ERCP. Of these, 7 had protein plugs in the common channel, and 2 had impacted sludges on the distal common bile duct. One patient had severe cholecystitis and underwent percutaneous transhepatic gallbladder drainage. One patient with protein plugs in the common channel developed pancreatitis after ERCP, but which improved after conservative treatment. Herein, all 9 patients who underwent ERCP underwent endoscopic sphincterotomy. Of the 5 group B patients who underwent preoperative biliary drainage, 1 underwent diagnostic ERCP without MRCP, and another underwent ERCP for cause analysis and treatment for severe pancreatitis. Patients aged 33, 25, and 18 months underwent percutaneous transhepatic biliary drainage for treating severe cholangiohepatitis, which extended to the intrahepatic duct. Generally, the reason why ERCP or PTC is not routinely performed in pediatric patients with CC is that these procedures generally require general anesthesia for pediatric patients and are invasive enough to cause bleeding, intestinal perforation, cholangitis, or pancreatitis [2324]. Because of the invasiveness of ERCP or PTC, MRCP is a very useful tool for diagnosing CCs. MRCP can visualize cysts and their surrounding anatomy and evaluate the presence of APBDU and the anatomy of the common channel [252627]. Preoperative therapeutic biliary drainage in patients with CC with persistent or exacerbated symptoms is considered to help make the surgery easier to perform and help them recover rapidly after surgery [2829]. A previous group that underwent biliary drainage through ERCP before surgery had significantly less intraoperative blood loss and transfusion and shorter postoperative length of stay than a group that did not [30]. Younger patients are known to have higher risks for complications with ERCP [3132]; however, recent studies have shown that the efficacy and safety of ERCP in infants and children are comparable to those in adults [23333435]. In our institution, ERCP has been actively performed in children since 2010 and in patients with persistent or exacerbated symptoms of CCs who do not respond to medical therapy before surgery.
As this study was conducted retrospectively, there might be some limitations. Although this study was conducted on patients with well-preserved records, it is true that there were many missing data in records, especially prior to 2000. This was particularly frequent in laboratory findings, for example, preoperative data on amylase or lipase were found in only 76 of 128 patients (59.4%) before 2000, and 250 of 262 patients (95.4%) after 2000. Therefore, 30.8% of patients diagnosed with accompanied pancreatitis in this study are likely to have been underestimated. Additionally, the lack of unified preoperative imaging tests to identify protein plugs is a limitation of this study. Also, if the surgeon did not pay attention to finding the protein plugs, it is likely that observer bias has occurred. We believe that MRCP is the best method for identifying protein plugs. Preoperative imaging evaluation with MRCP is noninvasive and can obtain accurate information on the pancreaticobiliary tract. Our center has recently actively performed preoperative MRCP. The 30-year preoperative MRCP rate at this institution is as follows: 5 of 141 patients (3.5%) in 1987–2000, 30 of 146 (20.5%) in 2001–2010, and 93 of 103 (90.3%) in 2011–2017. This increase is thought to be attributed to the ease of access to MRCP and the possibility of safe sedation. With more data obtained using MRCP, more features of CCs with protein plugs will be found. Although several patients have protein plugs, few studies have been conducted on their features. Herein, the enrollment of a large number of pediatric patients with CC who underwent surgery at a single center is of great significance.
In conclusion, of the pediatric patients with CC, 36.4% had protein plugs. Abdominal pain, pancreatitis, and APBDU were more commonly found among those with protein plugs than among those without. The presence of protein plugs had a similar proportion for types I and IVa, and the long-term complications did not differ. Through this study, we were able to identify the features of protein plugs in pediatric CCs, which would be of great help in managing pediatric patients with CC.
References
1. Ryu HS, Lee JY, Kim DY, Kim SC, Namgoong JM. Minimally-invasive neonatal surgery: laparoscopic excision of choledochal cysts in neonates. Ann Surg Treat Res. 2019; 97:21–26. PMID: 31297349.

2. Singham J, Yoshida EM, Scudamore CH. Choledochal cysts: part 2 of 3: diagnosis. Can J Surg. 2009; 52:506–511. PMID: 20011188.
3. Hung MH, Lin LH, Chen DF, Huang CS. Choledochal cysts in infants and children: experiences over a 20-year period at a single institution. Eur J Pediatr. 2011; 170:1179–1185. PMID: 21350805.

4. Lipsett PA, Pitt HA. Surgical treatment of choledochal cysts. J Hepatobiliary Pancreat Surg. 2003; 10:352–359. PMID: 14598135.

5. Lai HS, Duh YC, Chen WJ, Chen CC, Hung WT, Lee PH, et al. Manifestations and surgical treatment of choledochal cyst in different age group patients. J Formos Med Assoc. 1997; 96:242–246. PMID: 9136509.
6. Huang CS, Huang CC, Chen DF. Choledochal cysts: differences between pediatric and adult patients. J Gastrointest Surg. 2010; 14:1105–1110. PMID: 20422306.

7. Kaneko K, Ando H, Ito T, Watanabe Y, Seo T, Harada T, et al. Protein plugs cause symptoms in patients with choledochal cysts. Am J Gastroenterol. 1997; 92:1018–1021. PMID: 9177522.
8. Komuro H, Makino SI, Yasuda Y, Ishibashi T, Tahara K, Nagai H. Pancreatic complications in choledochal cyst and their surgical outcomes. World J Surg. 2001; 25:1519–1523. PMID: 11775184.

9. Fujishiro J, Masumoto K, Urita Y, Shinkai T, Gotoh C. Pancreatic complications in pediatric choledochal cysts. J Pediatr Surg. 2013; 48:1897–1902. PMID: 24074664.

10. Guelrud M, Morera C, Rodriguez M, Prados JG, Jaén D. Normal and anomalous pancreaticobiliary union in children and adolescents. Gastrointest Endosc. 1999; 50:189–193. PMID: 10425411.

11. Kim MJ, Han SJ, Yoon CS, Kim JH, Oh JT, Chung KS, et al. Using MR cholangiopancreatography to reveal anomalous pancreaticobiliary ductal union in infants and children with choledochal cysts. AJR Am J Roentgenol. 2002; 179:209–214. PMID: 12076938.

12. Saito T, Terui K, Mitsunaga T, Nakata M, Yoshida H. Significance of imaging modalities for preoperative evaluation of the pancreaticobiliary system in surgery for pediatric choledochal cyst. J Hepatobiliary Pancreat Sci. 2016; 23:347–352. PMID: 26994400.

13. Morinville VD, Husain SZ, Bai H, Barth B, Alhosh R, Durie PR, et al. Definitions of pediatric pancreatitis and survey of present clinical practices. J Pediatr Gastroenterol Nutr. 2012; 55:261–265. PMID: 22357117.

14. Suzuki M, Shimizu T, Kudo T, Suzuki R, Ohtsuka Y, Yamashiro Y, et al. Usefulness of nonbreath-hold 1-shot magnetic resonance cholangiopancreatography for the evaluation of choledochal cyst in children. J Pediatr Gastroenterol Nutr. 2006; 42:539–544. PMID: 16707978.

15. Kaneko K, Ando H, Seo T, Ono Y, Tainaka T, Sumida W. Proteomic analysis of protein plugs: causative agent of symptoms in patients with choledochal cyst. Dig Dis Sci. 2007; 52:1979–1986. PMID: 17415647.

16. Guy O, Robles-Diaz G, Adrich Z, Sahel J, Sarles H. Protein content of precipitates present in pancreatic juice of alcoholic subjects and patients with chronic calcifying pancreatitis. Gastroenterology. 1983; 84:102–107. PMID: 6401181.

17. Mariani A, Bernard JP, Provansal-Cheylan M, Nitsche S, Sarles H. Differences of pancreatic stone morphology and content in patients with pancreatic lithiasis. Dig Dis Sci. 1991; 36:1509–1516. PMID: 19160597.

18. Lee BI, Mustafi D, Cho W, Nakagawa Y. Characterization of calcium binding properties of lithostathine. J Biol Inorg Chem. 2003; 8:341–347. PMID: 12589570.

19. Patard L, Lallemand JY, Stoven V. An insight into the role of human pancreatic lithostathine. JOP. 2003; 4:92–103. PMID: 12629266.
20. Diao M, Li L, Cheng W. Congenital biliary dilatation may consist of 2 disease entities. J Pediatr Surg. 2011; 46:1503–1509. PMID: 21843715.

21. Sugiyama M, Atomi Y, Kuroda A. Pancreatic disorders associated with anomalous pancreaticobiliary junction. Surgery. 1999; 126:492–497. PMID: 10486601.

22. Komi N, Takehara H, Kunitomo K, Miyoshi Y, Yagi T. Does the type of anomalous arrangement of pancreaticobiliary ducts influence the surgery and prognosis of choledochal cyst? J Pediatr Surg. 1992; 27:728–731. PMID: 1306647.

23. Saito T, Terui K, Mitsunaga T, Nakata M, Kuriyama Y, Higashimoto Y, et al. Role of pediatric endoscopic retrograde cholangiopancreatography in an era stressing less-invasive imaging modalities. J Pediatr Gastroenterol Nutr. 2014; 59:204–209. PMID: 24762457.

24. Soares KC, Goldstein SD, Ghaseb MA, Kamel I, Hackam DJ, Pawlik TM. Pediatric choledochal cysts: diagnosis and current management. Pediatr Surg Int. 2017; 33:637–650. PMID: 28364277.

25. Edil BH, Olino K, Cameron JL. The current management of choledochal cysts. Adv Surg. 2009; 43:221–232. PMID: 19845181.

26. Soares KC, Arnaoutakis DJ, Kamel I, Rastegar N, Anders R, Maithel S, et al. Choledochal cysts: presentation, clinical differentiation, and management. J Am Coll Surg. 2014; 219:1167–1180. PMID: 25442379.

27. Park DH, Kim MH, Lee SK, Lee SS, Choi JS, Lee YS, et al. Can MRCP replace the diagnostic role of ERCP for patients with choledochal cysts? Gastrointest Endosc. 2005; 62:360–366. PMID: 16111952.

28. Terui K, Yoshida H, Kouchi K, Hishiki T, Saito T, Mitsunaga T, et al. Endoscopic sphincterotomy is a useful preoperative management for refractory pancreatitis associated with pancreaticobiliary maljunction. J Pediatr Surg. 2008; 43:495–499. PMID: 18358288.

29. Tsuchiya H, Kaneko K, Itoh A, Kawashima H, Ono Y, Tainaka T, et al. Endoscopic biliary drainage for children with persistent or exacerbated symptoms of choledochal cysts. J Hepatobiliary Pancreat Sci. 2013; 20:303–306. PMID: 22581057.

30. Wang Q, Moon SB, Zang J, Liu J, Weng H, Wang X, et al. Usefulness of pre-operative endoscopic retrograde cholangiopancreatography in diagnosis and management of forme fruste choledochal cyst in children. ANZ J Surg. 2020; 90:1041–1045. PMID: 31943672.

31. Limketkai BN, Chandrasekhara V, Kalloo AN, Okolo PI 3rd. Comparison of performance and safety of endoscopic retrograde cholangiopancreatography across pediatric age groups. Dig Dis Sci. 2013; 58:2653–2660. PMID: 23709156.

32. Rosen JD, Lane RS, Martinez JM, Perez EA, Tashiro J, Wagenaar AE, et al. Success and safety of endoscopic retrograde cholangiopancreatography in children. J Pediatr Surg. 2017; 52:1148–1151. PMID: 28188033.

33. Keil R, Drábek J, Lochmannová J, Šťovíček J, Koptová P, Wasserbauer M, et al. ERCP in infants, children, and adolescents: different roles of the methods in different age groups. PLoS One. 2019; 14:e0210805. PMID: 30653580.
34. De Angelis P, Foschia F, Romeo E, Caldaro T, Rea F, di Abriola GF, et al. Role of endoscopic retrograde cholangiopancreatography in diagnosis and management of congenital choledochal cysts: 28 pediatric cases. J Pediatr Surg. 2012; 47:885–888. PMID: 22595566.

35. Yıldırım AE, Altun R, Ocal S, Kormaz M, Ozcay F, Selcuk H. The safety and efficacy of ERCP in the pediatric population with standard scopes: does size really matter? Springerplus. 2016; 5:128. PMID: 26933627.

Fig. 1
Protein plug in the pancreaticobiliary duct. (A) Operative cholangiographic image of a 13-year-old girl showing multiple protein plugs (arrows) in the left intrahepatic duct, common hepatic duct, and distal common bile duct. (B) T2-weighted magnetic resonance cholangiopancreatography (MRCP) image of a 22-month-old boy showing filling defects (arrow) in the distal common bile duct. (C) T2-weighted MRCP image of an 8-year-old girl showing multiple filling defects (arrows) in the common channel.




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download