1. Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examinat ion Sur vey, 1999-2000. Circulation. 2004; 110:738–743. PMID:
15262830.
2. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur J Vasc Endovasc Surg. 2007; 33 Suppl 1:S1–S75. PMID:
17140820.

3. Upchurch GR, Dimick JB, Wainess RM, Eliason JL, Henke PK, Cowan JA, et al. Diffusion of new technology in health care: the case of aorto-iliac occlusive disease. Surgery. 2004; 136:812–818. PMID:
15467666.

4. Goodney PP, Beck AW, Nagle J, Welch HG, Zwolak RM. National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. J Vasc Surg. 2009; 50:54–60. PMID:
19481407.

5. Groot Jebbink E, Holewijn S, Versluis M, Grimme F, Hinnen JW, Sixt S, et al. Meta-analysis of individual patient data after kissing stent treatment for aortoiliac occlusive disease. J Endovasc Ther. 2019; 26:31–40. PMID:
30499352.

6. Björses K, Ivancev K, Riva L, Manjer J, Uher P, Resch T. Kissing stents in the aortic bifurcation: a valid reconstruction for aorto-iliac occlusive disease. Eur J Vasc Endovasc Surg. 2008; 36:424–431. PMID:
18692412.
7. Haulon S, Mounier-Véhier C, Gaxotte V, Koussa M, Lions C, Haouari BA, et al. Percutaneous reconstruction of the aortoiliac bifurcation with the “kissing stents” technique: long-term follow-up in 106 patients. J Endovasc Ther. 2002; 9:363–368. PMID:
12096953.

8. Park KB, Do YS, Kim DI, Kim DK, Kim YW, Shin SW, et al. The TransAtlantic Inter Society Consensus (TASC) classification system in iliac arterial stent placement: long-term patency and clinical limitations. J Vasc Interv Radiol. 2007; 18:193–201. PMID:
17327551.
9. de Vries SO, Hunink MG. Results of aortic bifurcation grafts for aortoiliac occlusive disease: a meta-analysis. J Vasc Surg. 1997; 26:558–569. PMID:
9357455.

10. Nyman U, Uher P, Lindh M, Lindblad B, Ivancev K. Primary stenting in infrarenal aortic occlusive disease. Cardiovasc Intervent Radiol. 2000; 23:97–108. PMID:
10795833.

11. Mouanoutoua M, Maddikunta R, Allaqaband S, Gupta A, Shalev Y, Tumuluri R, et al. Endovascular intervention of aortoiliac occlusive disease in high-risk patients using the kissing stents technique: long-term results. Catheter Cardiovasc Interv. 2003; 60:320–326. PMID:
14571480.

12. Pulli R, Dorigo W, Fargion A, Angiletta D, Azas L, Pratesi G, et al. Early and midterm results of kissing stent technique in the management of aortoiliac obstructive disease. Ann Vasc Surg. 2015; 29:543–550. PMID:
25595108.

13. Sixt S, Krankenberg H, Möhrle C, Kaspar M, Tübler T, Rastan A, et al. Endovascular treatment for extensive aortoiliac artery reconstruction: a single-center experience based on 1712 interventions. J Endovasc Ther. 2013; 20:64–73. PMID:
23391085.

14. Farb A, Sangiorgi G, Carter AJ, Walley VM, Edwards WD, Schwartz RS, et al. Pathology of acute and chronic coronary stent ing in humans. Circulation. 1999; 99:44–52. PMID:
9884378.
15. AbuRahma AF, Hayes JD, Flaherty SK, Peery W. Primary iliac stenting versus transluminal angioplasty with selective stenting. J Vasc Surg. 2007; 46:965–970. PMID:
17905559.

16. Ozkan U, Oguzkurt L, Tercan F. Technique, complication, and long-term outcome for endovascular treatment of iliac artery occlusion. Cardiovasc Intervent Radiol. 2010; 33:18–24. PMID:
19768500.

17. Ichihashi S, Higashiura W, Itoh H, Sakaguchi S, Nishimine K, Kichikawa K. Long-term outcomes for systematic primary stent placement in complex iliac artery occlusive disease classified according to Trans-Atlant ic Inter-Society Consensus (TASC)-II. J Vasc Surg. 2011; 53:992–999. PMID:
21215582.
18. Rzucidlo EM, Powell RJ, Zwolak RM, Fillinger MF, Walsh DB, Schermerhorn ML, et al. Early results of stent-grafting to treat diffuse aortoiliac occlusive disease. J Vasc Surg. 2003; 37:1175–1180. PMID:
12764261.

19. Houston JG, Bhat R, Ross R, Stonebridge PA. Long-term results after placement of aortic bifurcation self-expanding stents: 10 year mortality, stent restenosis, and distal disease progression. Cardiovasc Intervent Radiol. 2007; 30:42–47. PMID:
17122886.

20. Palmaz JC. Intravascular stents: tissue-stent interactions and design considerations. AJR Am J Roentgenol. 1993; 160:613–618. PMID:
8430566.

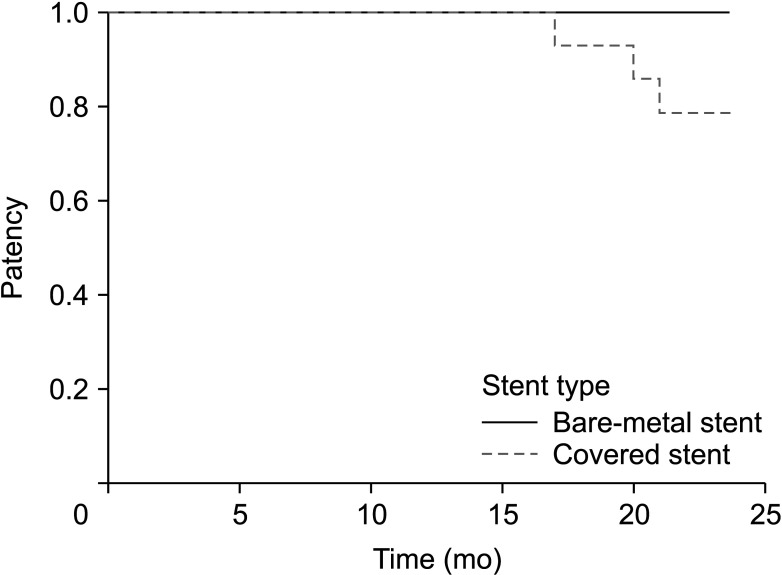
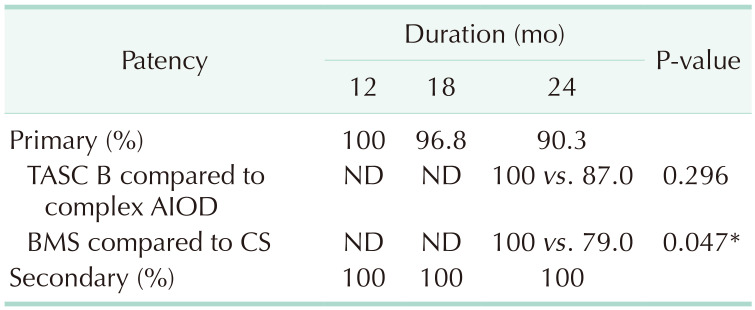
21. Sabri SS, Choudhri A, Orgera G, Arslan B, Turba UC, Harthun NL, et al. Outcomes of covered kissing stent placement compared with bare metal stent placement in the treatment of atherosclerotic occlusive disease at the aortic bifurcation. J Vasc Interv Radiol. 2010; 21:995–1003. PMID:
20538478.

22. Mwipatayi BP, Sharma S, Daneshmand A, Thomas SD, Vijayan V, Altaf N, et al. Durability of the balloon-expandable covered versus bare-metal stents in the Covered versus Balloon Expandable Stent Trial (COBEST) for the treatment of aortoiliac occlusive disease. J Vasc Surg. 2016; 64:83–94. PMID:
27131926.

23. Hinnen JW, Konickx MA, Meerwaldt R, Kolkert JL, van der Palen J, Huisman AB, et al. Long term results of kissing stents in the aortic bifurcation. Acta Chir Belg. 2015; 115:191–197. PMID:
26158249.

24. de Donato G, Bosiers M, Setacci F, Deloose K, Galzerano G, Verbist J, et al. 24-Month data from the BRAVISSIMO: a large-scale prospective registry on iliac stenting for TASC A & B and TASC C & D lesions. Ann Vasc Surg. 2015; 29:738–750. PMID:
25733220.
25. Saker MB, Oppat WF, Kent SA, Ryu RK, Chrisman HB, Nemcek AA, et al. Early failure of aortoiliac kissing stents: histopathologic correlation. J Vasc Interv Radiol. 2000; 11:333–336. PMID:
10735428.

26. Vértes M, Juhász IZ, Nguyen TD, Veres DS, Hüttl A, Nemes B, et al. Stent protrusion >20 mm into the aorta: a new predictor for restenosis after kissing stent reconstruction of the aortoiliac bifurcation. J Endovasc Ther. 2018; 25:632–639. PMID:
30122138.
27. Piffaretti G, Fargion AT, Dorigo W, Pulli R, Gattuso A, Bush RL, et al. Outcomes from the multicenter italian registry on primary endovascular treatment of aortoiliac occlusive disease. J Endovasc Ther. 2019; 26:623–632. PMID:
31331235.

28. Taeymans K, Groot Jebbink E, Holewijn S, Martens JM, Versluis M, Goverde PCJM, et al. Three-year outcome of the covered endovascular reconstruction of the aortic bifurcation technique for aortoiliac occlusive disease. J Vasc Surg. 2018; 67:1438–1447. PMID:
29169878.

29. Mwipatayi BP, Thomas S, Wong J, Temple SE, Vijayan V, Jackson M, et al. A comparison of covered vs bare expandable stents for the treatment of aortoiliac occlusive disease. J Vasc Surg. 2011; 54:1561–1570. PMID:
21906903.

30. Noori VJ, Eldrup-Jørgensen J. A systematic review of vascular closure devices for femoral artery puncture sites. J Vasc Surg. 2018; 68:887–899. PMID:
30146036.

31. Das R, Ahmed K, Athanasiou T, Morgan RA, Belli AM. Arterial closure devices versus manual compression for femoral haemostasis in interventional radiological procedures: a systematic review and meta-analysis. Cardiovasc Intervent Radiol. 2011; 34:723–738. PMID:
21046386.





 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download