Abstract
Purpose
Intrathecal analgesia (ITA) and transverse abdominis plane block (TAPB) are effective pain control methods in abdominal surgery. However, there is still no gold standard for postoperative pain control in minimally invasive colorectal surgery. This study aimed to investigate whether the analgesic effect could be increased when TAPB, which can further reduce wound somatic pain, was administered in low-dose morphine ITA patients.
Methods
Patients undergoing elective colorectal surgery were randomized into an ITA with TAPB group or an ITA group. Patients were evaluated for pain 0, 8, 16, 24, and 48 hours after surgery. The primary outcome was the total morphine milligram equivalents administered 24 hours after surgery. The secondary outcomes were pain scores, ambulatory variables, inflammation markers, hospital stay duration, and complications within 48 hours after surgery.
Results
A total of 64 patients were recruited, and 55 were compared. There was no significant difference in morphine use over the 24 hours after surgery in the 2 groups (ITA with TAPB, 15.3 mg vs. ITA, 10.2 mg; P = 0.270). Also, there was no significant difference in pain scores. In both groups, the average pain score at 24 and 48 hours was 2 points or less, showing effective pain control.
Effective postoperative pain control is an important factor in patient recovery. The effects of perioperative multimodal pain control with regional anesthesia are well known to be reduced surgical stress response and enhanced recovery [1]. thoracic epidural analgesia for open colorectal surgery is a standard pain management guideline for enhanced recovery after surgery (ERAS), it is not strongly recommended for laparoscopic surgery due to its poor success rate and side effects such as urinary retention [1]. In recent ERAS guidelines for colorectal surgery, diverse regional analgesic methods such as a transverse abdominis plane block (TAPB), intrathecal analgesia (ITA), and a rectus sheath block have been introduced, and their good effects have been reported in laparoscopic abdominal surgery [123]. In addition, comparative studies on each of the regional anesthesia methods are being conducted, but there are few comparative studies in patients with minimally invasive colorectal surgery, so the ERAS guideline does not yet recommend which regional analgesic method is the most effective [245678].
In a recent report on colorectal surgical patients, a randomized controlled study comparing the TAPB and ITA demonstrated that the opioid-sparing effect of ITA within 24 hours after surgery was better than that of the TAPB [4]. Another study reported a higher pain relief effect of TAPB than that of epidural block in patients who underwent minimally invasive colorectal surgery [6]. Before this study, in our pilot study, we observed the lowest postoperative pain score and opioid consumption in patients who received both TAPB and ITA. Therefore, to identify whether a multi-block regional anesthesia technique with different mechanisms of effect could result in the best pain relief and opioid-sparing effects in patients who underwent minimally invasive colorectal surgery, a randomized controlled comparison between ITA and ITA with TAPB was conducted.
This study was to compare postoperative pain relief effects in patients who underwent elective minimal invasive colorectal surgery in a prospective, single-blinded, randomized controlled trial with groups that received either ITA alone or both ITA and laparoscopic TAPB. This study was conducted from December 2019 to October 2020 in Seoul St. Mary’s Hospital, the Catholic University of Korea. The study was approved by the Institutional Review Board of the Ethics Committee of the College of Medicine, the Catholic University of Korea (No. KC19MISV0923) and was registered at the Clinical Trial Registry of Korea (No. KCT0004838). This study was performed in accordance with the Declaration of Helsinki and written informed consent was obtained from all the patients.
Patients scheduled to undergo elective, minimally invasive colorectal surgery in Seoul St. Mary’s Hospital, the Catholic University of Korea were recruited for this study. Inclusion criteria were adults under 80 years old and American Society of Anesthesiologists physical status classifications I–II; colorectal cancer or benign disease except for inflammatory bowel diseases such as Crohn disease and ulcerative colitis. Exclusion criteria were women who were pregnant or breastfeeding; chronic patients requiring daily opioid use; abnormal liver function test results; renal insufficiency, defined as preoperative serum creatinine > 2.0 mg/dL; synchronous surgery on another organ; abdominoperineal resection; rectal cancer patients who underwent preoperative chemoradiotherapy; or a history of side effects of spinal anesthesia or spinal surgery. In accordance with the American Society of Regional Anesthesia and Pain Medicine Guidelines, any patients receiving any anticoagulants other than aspirin were also excluded.
All patients, after providing written informed consent and satisfying the criteria, were allocated to a study group. Hospital staff who were not involved in this study performed computer-generated randomization at a 1:1 ratio to allocate the enrolled patients to the ITA with TAPB group (n = 29) or the ITA-only group (n = 30). The randomized allocation was performed on the day when a patient who gave informed consent was admitted.
All patients were managed pre-, intra-, and postoperatively according to our institution’s multimodal pain management protocol. Patients received oral medications (900 mg of acetaminophen, 200 mg of celecoxib, and 300 mg of gabapentin) before surgery, and in the operating room, patients were treated with ITA with or without TAPB according to their group allocations and received intravenous injections of 2 g of acetaminophen with ketorolac 30 mg before the end of anesthesia. Postoperatively, intravenous patient-controlled analgesia (PCA) was applied; the intravenous PCA regimen consisted of fentanyl 2,000 µg (total of 100 mL of volume including saline), and the device was programmed to deliver 1 mL (20 µg of fentanyl) per demand without basal infusion. Additional pain control was performed in the order of ketorolac, tramadol, and pethidine.
Spinal analgesia was performed in the operating room before the induction of general anesthesia by 2 anesthesiologists (SHH and JWS). For this, 0.5% hyperbaric bupivacaine 10 mg and preservative-free morphine were administered, with the patient in the lateral position, at the L3–4 or L4–5 intervertebral space using a 25-gauge Quinke needle. Male patients aged ≥70 years and female patients received 150 µg of intrathecal morphine, and male patients aged <70 years received 200 µg of intrathecal morphine.
The laparoscopic TAPB was modified from the TAPB described in a previous study [9]. All laparoscopic TAPBs were conducted with ropivacaine hydrochloride before the wound was closed. Ropivacaine solution (0.75%, 150 mg in 20 mL) was diluted with 20 mL saline to a total volume of 40 mL (0.375%, 150 mg in 40 mL). Ropivacaine was injected into the transverse abdominis plane from the peritoneal side under ultrasound guidance. Under ultrasonic visualization of the transverse abdominis plane between the internal oblique and transverse abdominis muscles, the surgeons introduced an 18-gauge laparoscopic needle into the plane between the lower costal margin and the iliac crest, injecting 20 mL of diluted ropivacaine on each side [9]. Each laparoscopic TAPB was performed by 3 surgeons participating in the surgery, securing intestinal safety with a laparoscopic camera. The plane positions of all TAP blocks were cross-confirmed by other surgeons.
Laparoscopic surgery was performed using 4 trocars with a small wound protector in the umbilicus, where the specimen was extracted by transumbilical incision. With robotic surgery, 4 to 5 trocars with a small wound protector were used, and the specimen was extracted through wound protector placed in a transumbilical or right lower quadrant. Complete mesocolic excision with central vascular ligation in colon surgery or total mesorectal excision in rectal surgery was performed according to the tumor location. All patients underwent double stapled anastomosis by side-to-end anastomosis in right hemicolectomy or end-to-end anastomosis in left colon and rectal surgery.
The primary outcome was the total morphine milligram equivalents (MMEs) administered within the first 24 hours after surgery. Secondary outcomes were mean pain scores within 24 and 48 hours after surgery, the time for the first ambulation, ambulatory distances within 24 and 48 hours, postoperative inflammatory markers, and clinical outcomes. Pain was assessed on a visual analogue scale ranging from 0 (no pain) to 10 (severe pain) by a specialized ERAS nurse unaware of allocation at 0, 8, 16, 24, and 48 hours after surgery. All patients received perioperative care from an ERAS nurse before and after the surgery. When they arrived at the ward after the surgery, each patient wore a smart bracelet (InBodyBAND2, InBody Corp., Seoul, Korea), and the data on the patient’s postoperative exercise were stored in the application installed on the patient’s own smartphone. In this study, the time until the patient’s first ambulation and the number of walking steps within 24 and 48 hours after surgery were investigated by smart devices. Markers of inflammation were evaluated daily for 48 hours after surgery, including serum albumin, CRP, ESR, WBC count, neutrophil and lymphocyte counts, and the neutrophil to lymphocyte ratio (NLR). A modified Glasgow prognostic score (mGPS), which is based on serum CRP and albumin concentrations, was also determined. The NLR and mGPS were used as systemic inflammation markers. NLR was classified as higher or lower than 5, and mGPS was divided into stages 0, 1, and 2. Higher NLR and higher mGPS values indicate severe inflammation. In addition, we investigated patient hospital stay and surgical complications.
The sample size was calculated based on the primary outcome according to the superiority hypothesis. Based on preliminary analyses (unpublished), the 24-hour MMEs of the ITA and TAPB group (n = 18) and the ITA-only group (n = 5) were 17.1 vs. 24.3, and the standard deviation was 11 MMEs. With α = 0.05 and a desired power of 80%, assuming a 10% dropout rate, we decided to enroll 32 patients per group.
This primary analysis was performed on per-protocol basis with a secondary intention-to-treat (ITT) analysis. The categorical variables of the groups were compared using the chi-square or Fisher exact test. The chi-square and Pearson correlation tests were used to evaluate the correlations between clinical factors. We used the 2-sided independent sample Mann-Whitney test or t-test for the continuous variables of opioid consumption (MMEs) and pain scores. All statistical analyses were performed using IBM SPSS for Windows ver. 24.0 (IBM Corp., Armonk, NY, USA). P-values less than 0.05 were considered significant.
A total of 59 patients who were eligible according to our criteria were enrolled in this study. Four patients withdrew after randomization because of 2 failures of ITA, an open conversion, and an open and closure due to carcinomatosis. Ultimately, 55 patients were included and allocated in the ITA with laparoscopic TAPB group (n = 27) and the ITA-only group (n = 28) (Fig. 1). Baseline demographic and clinical characteristics with preoperative inflammatory status are shown in Table 1. There was no significant difference between the 2 groups among the preoperative inflammatory markers. The main results, primary and secondary outcomes were presented in Table 2 for the per-protocol with ITT analysis.
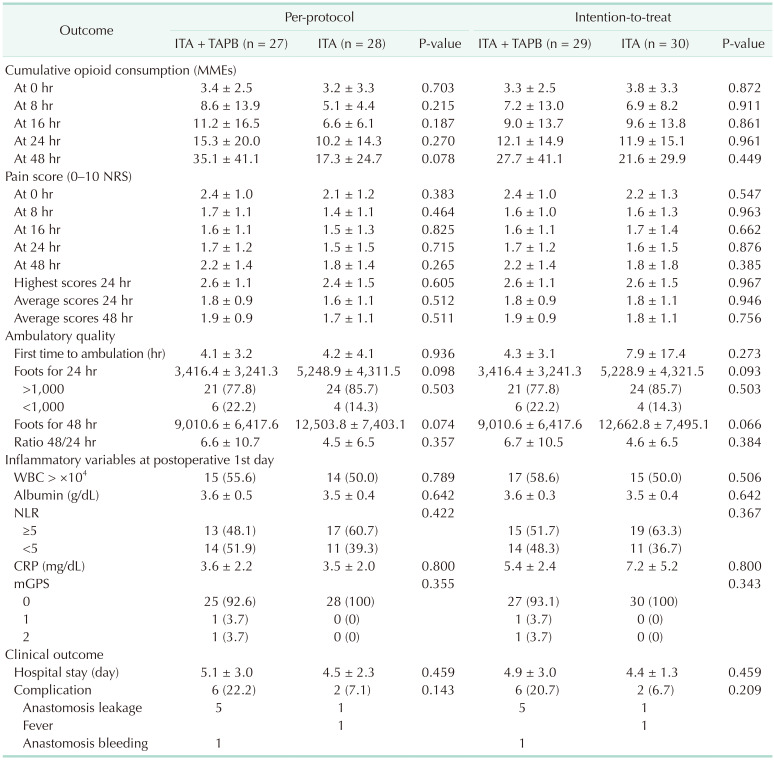
The primary and secondary outcomes are shown in Table 2. In per-protocol analysis, the total MMEs administered within the first 24 hours after surgery were 15.3 ± 20.0 mg in the ITA with laparoscopic TAPB group and 10.2 ± 14.3 mg in the ITA-only group (P = 0.270). There was no significant difference in opioid consumption between the 2 groups for 24 hours after surgery (Fig. 2). In the ITT analysis, we could not find significant difference in opioid consumption between the 2 groups for 24 hours after surgery (Table 2).
In per-protocol analysis, 1 of the secondary outcomes, the mean pain scores within 24 hours and 48 hours, showed no differences between the 2 groups (24 hours: 1.8 ± 0.9 vs. 1.6 ± 1.1, P = 0.512; 48 hours: 1.9 ± 0.9 vs. 1.7 ± 1.1, P = 0.511). Both groups started ambulation about 4.2 hours after surgery, and there were no significant differences between the 2 groups with regard to inflammatory changes measured after surgery. The duration of hospital stay showed no significant difference between the 2 groups (5.1 ± 3.0 days vs. 4.5 ± 2.3 days, P = 0.459). However, the ITA and laparoscopic TAPB group showed a trend of higher postoperative complications than the ITA group, although this was without statistical significance (22.2% vs. 7.1%, P = 0.143). In the ITT analysis, we could not find significant difference in pain scores within 24 and 48 hours, ambulatory quality, hospital stay, and inflammatory variables between the 2 groups (Table 2).
One patient had a serious adverse event during this study. The patient was not included in the analysis due to ITA failure, but a reoperation was performed due to external iliac artery bleeding. In the ITA with laparoscopic TAPB group, 5 patients showed clinical symptoms indicative of anastomosis leakage and were treated with antibiotics and conservative management, and 1 patient had anastomosis site bleeding with conservative care. In contrast, in the ITA-only group, one patient was suspected of anastomosis minor leakage, so antibiotics and conservative care were provided (Table 2).
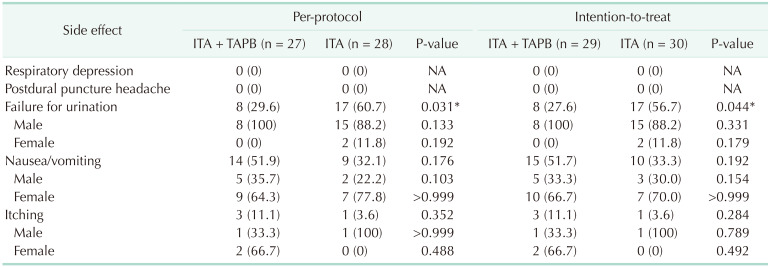
Table 3 summarizes the side effects associated with the interventions. There were no serious cardiovascular or respiratory problems. The urination failure was significantly higher in the ITA-only group (29.6% vs. 60.7%, P = 0.031). Of the 28 male patients in both groups, 23 patients (82.1%) had a Foley catheter reinserted after surgery. Among the 27 female patients, 16 patients (59.3%) complained of nausea and vomiting (Supplementary Fig. 1).
In the present study, we found that the patients who underwent ITA showed effective pain control with a pain score of 2 points or less until 48 hours after surgery. However, additional TAPB in patients with ITA for laparoscopic colorectal surgery had no additional analgesic effects. The use of ITA alone provided satisfactory analgesia.
According to the previous literature, both ITA and TAPB showed significant pain relief effects in patients who underwent laparoscopic abdominal surgery as pain control methods with relatively low complications [4610111213]. In this study, it was also confirmed that effective pain control was achieved in all patients who underwent ITA with or without TAPB. It was confirmed that the average pain score within 24 and 48 hours after surgery was maintained at 2 points or less in both groups, and the amounts of opioid consumption were 15.3 and 10.2 mg within 24 hours, respectively. In laparoscopic abdominal surgeries where ITA was performed in other institutions, opioid usage was 11.3–22.5 mg, and pain score was 0.3–2.8 points, similar to our results [4111415].
Since 1979, ITA, which has been used for postoperative pain control, has been used at various effective doses of 0.1–2.5 mg [16]. However, there have been reports related to side effects such as pruritus, nausea, vomiting, and respiratory failure. Therefore, in recent years, it has become important to use a small amount of morphine to achieve adequate pain control. Many studies have recently reported the use of low-dose morphine from abdominal surgery to orthopedic surgery, confirming its effectiveness [121718]. It has been reported that dosages of morphine used in ITA of abdominal surgery ranged between 0.1 and 0.8 mg, and there were some studies in which 0.015–0.05 mg/kg was used as a weight-adjusted dose [12]. Fares et al. [19] reported on the relationship between morphine dose and the effect of pain control. According to this study, the group using 1 mg of morphine reported a longer pain effect and an opioid reduction effect in turn, compared to the groups using 0.5 and 0.2 mg. There is a dilemma in that using small doses of morphine decreases pain control effects with fewer side effects, with no current consensus on a low dosage for morphine.
Until now, only a few multiple nerve block techniques have been attempted during abdominal surgery. According to these studies conducted in Cesarean sections and laparoscopic colorectal surgery, the groups using ITA and TAPB reported better pain relief within 24 hours postoperatively than those using TAPB alone [1520]. In orthopedic surgery, when the femoral nerve block and ITA were performed together, a significant pain reduction effect was reported compared to when a single pain modality was used [21]. Therefore, this study was planned under the hypothesis that the analgesic effects of the low dose of morphine used for ITA could be enhanced in the early postoperative period by blocking the conduction of somatic pain with an additional TAPB.
However, in this study, no additional effect could be confirmed when adding TAPB in patients who underwent ITA. Above all, since the patients in this study were colorectal patients who underwent laparoscopic surgery, their abdominal incision was not large and the degree of pain was less severe than that of open surgery, so the dependence on somatic pain control may have been low. The comparison of postoperative pain between open and laparoscopic surgery patients has proven the better postoperative pain relief of laparoscopic surgery in many studies [22].
The second reason is that even with the dose of 150–200 µg of intrathecal morphine used in this study, a sufficient pain control effect can be obtained. So far, the minimum dose of intrathecal morphine used in colorectal surgery was 200 µg, as in our study. Even in these studies, the pain control effect was better than that of the control group [1523]. After intrathecal injection of morphine, the morphine is circulated with cerebrospinal fluids (CSF) in the intrathecal space and is diffused into the spinal cord. For proper ITA, the injected morphine acts only on the opioid receptors in the dorsal horn of the spinal cord from the CSF, providing somatic and visceral pain relief. Therefore, it is known that excessive amounts of morphine are redistributed to the brain by rostral spread in CSF, causing a dose-limiting side effect [24]. In contrast, after reaching the concentration causing the analgesic effect, the pain relief effect according to the morphine dose appears not dose-dependent, but time-dependent. Therefore, the initial pain-reducing effects of high-dose and low-dose morphine were similar, but the time to first request for rescue analgesics was longer in the high-dose group [171925]. Rather, it would have been possible to expect an additional TAPB effect using the long-acting liposomal agent as a time-dependent approach, and if a long-acting agent is imported into Korea, further research is needed.
Finally, the patients in this study were controlling their pain to some extent through oral multimodal pain control, so the effect of the TAPB may not have been significant. One study reported that there was no effect of the additional TAPB in patients who underwent oral multimodal pain control and received Cesarean sections [26].
Contrary to expectations, the group that received ITA and TAPB showed higher pain scores and opioid usage than the ITA-only group, although this was not statistically significant. This could be interpreted as a result of more complications such as anastomosis leakage in the ITA and TAPB groups. In the comparison between the 2 groups, excluding patients with complications, we observed that the patient group who underwent the additional TAPB, as we expected, used fewer opioids (8.2 mg vs. 10.8 mg), although this difference was not statistically significant (Supplementary Table 1).
Side effects commonly reported with the ITA are nausea, vomiting, pruritus, urinary retention, and respiratory depression [16]. According to one meta-analysis, there were more respiratory problems and a higher incidence of pruritus in studies using high-dose morphine (≥0.3 mg), whereas the studies that used low-dose morphine (<0.3 mg) reported nausea, vomiting, and pruritus as the main side effects [16]. In this study, there no respiratory depression occurred, and pruritus also had a low incidence, but nausea and vomiting occurred in 23 patients (41.8%), and a high urinary retention rate (45.5%) was shown. Patients with side effects showed significantly different characteristics according to sex. Nausea and vomiting occurred in 16 female patients (59.3%) compared to 7 male patients (25.0%) (P = 0.020), and urinary retention could be observed in male patients (82.1%, P < 0.001) (Supplementary Fig. 1). Previous studies reported an increased incidence of urinary retention in patients who had received intrathecal opioid administration [27]. According to the report of Kuipers et al. [28], opioids entering the subarachnoid dose-dependently reduced bladder contractility and urge sensation, and the recovery time of patients using 0.1 mg of morphine was reported as a maximum of 12 hours. All patients whose Foley catheters were reinserted in this study succeeded in urination 12 hours after the Foley catheter was removed. The reason that urinary retention in our study was somewhat higher is that Foley catheters are removed in the operating room before awakening from general anesthesia, according to the ERAS protocol of this institution. Although there are studies reporting that early Foley catheter removal is possible in patients who underwent ITA [29], this study using the earliest protocol for removal of Foley catheters observed a high rate of urinary retention.
This study has some limitations. It was conducted as a prospective randomized study, but the researchers were not blinded. However, for the patients and the nurse who assessed the pain scores, the allocation result was blinded, and the pain evaluation was objectively performed. In addition, there may be a risk of selection bias, because the laparoscopic and robotic surgeries of the patients in this study were not unified according to the operator.
Nevertheless, this study aimed to resolve whether additional analgesia to somatic pain has benefits in patients who underwent ITA using low-dose morphine. Although there were no severe side effects such as respiratory depression, this study provided additional information that different side effects prevail depending on the sex. In the future, based on the results of this study, it would be possible to conduct an additional large-scale study on whether additional TAPB can bring pain relief in large-incision surgeries such as open surgery or when long-acting TAPB agents are used.
In minimally invasive colorectal surgery, lower dose ITA was effective in alleviating pain, and an additional TAPB for additional pain relief was not observed. However, side effects such as nausea, vomiting, and urinary retention were still observed, so when using ITA, it is necessary to note the appropriate indications and provide preventive management against side effects.
ACKNOWLEDGEMENTS
I would like to express my gratitude to Soo-Ji Park, M.N., Seoul St. Mary's Hospital, who worked hard to evaluate the pain of patients after surgery as an ERAS nurse.
References
1. Gustafsson UO, Scott MJ, Hubner M, Nygren J, Demartines N, Francis N, et al. Guidelines for perioperative care in elective colorectal surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations: 2018. World J Surg. 2019; 43:659–695. PMID: 30426190.

2. Levy BF, Scott MJ, Fawcett W, Fry C, Rockall TA. Randomized clinical trial of epidural, spinal or patient-controlled analgesia for patients undergoing laparoscopic colorectal surgery. Br J Surg. 2011; 98:1068–1078. PMID: 21590762.

3. Kim WJ, Mun JY, Kim HJ, Yoon SH, Han SR, Bae JH, et al. Surgical rectus sheath block combined with multimodal pain management reduces postoperative pain and analgesic requirement after single-incision laparoscopic appendectomy: a retrospective study. Int J Colorectal Dis. 2021; 36:75–82. PMID: 32875376.

4. Colibaseanu DT, Osagiede O, Merchea A, Ball CT, Bojaxhi E, Panchamia JK, et al. Randomized clinical trial of liposomal bupivacaine transverse abdominis plane block versus intrathecal analgesia in colorectal surgery. Br J Surg. 2019; 106:692–699. PMID: 30919948.

5. Dewinter G, Coppens S, Van de Velde M, D’Hoore A, Wolthuis A, Cuypers E, et al. Quadratus lumborum block versus perioperative intravenous lidocaine for postoperative pain control in patients undergoing laparoscopic colorectal surgery: a prospective, randomized, double-blind controlled clinical trial. Ann Surg. 2018; 268:769–775. PMID: 30004914.
6. Felling DR, Jackson MW, Ferraro J, Battaglia MA, Albright JJ, Wu J, et al. Liposomal bupivacaine transversus abdominis plane block versus epidural analgesia in a colon and rectal surgery enhanced recovery pathway: a randomized clinical trial. Dis Colon Rectum. 2018; 61:1196–1204. PMID: 30192328.

7. Park JS, Choi GS, Kwak KH, Jung H, Jeon Y, Park S, et al. Effect of local wound infiltration and transversus abdominis plane block on morphine use after laparoscopic colectomy: a nonrandomized, single-blind prospective study. J Surg Res. 2015; 195:61–66. PMID: 25604485.

8. Xu YJ, Sun X, Jiang H, Yin YH, Weng ML, Sun ZR, et al. Randomized clinical trial of continuous transversus abdominis plane block, epidural or patient-controlled analgesia for patients undergoing laparoscopic colorectal cancer surgery. Br J Surg. 2020; 107:e133–e141. PMID: 31903600.

9. Lee CS, Park SJ, Hong SH, Shim JW, Chae MS, Han SR, et al. Clinical effect of multimodal perioperative pain management protocol for minimally invasive colorectal cancer surgery: propensity score matching study. Asian J Surg. 2021; 44:471–475. PMID: 33223452.

10. Wu RC, Jensen CC, Douaiher J, Madoff RD, Kwaan MR. Transversus abdominis plane block in laparoscopic colorectal surgery: a systematic review. Dis Colon Rectum. 2019; 62:1248–1255. PMID: 31490834.

11. Koning MV, Teunissen AJ, van der Harst E, Ruijgrok EJ, Stolker RJ. Intrathecal morphine for laparoscopic segmental colonic resection as part of an enhanced recovery protocol: a randomized controlled trial. Reg Anesth Pain Med. 2018; 43:166–173. PMID: 29219935.
12. Koning MV, Klimek M, Rijs K, Stolker RJ, Heesen MA. Intrathecal hydrophilic opioids for abdominal surgery: a meta-analysis, meta-regression, and trial sequent ial analysis. Br J Anaesth. 2020; 125:358–372. PMID: 32660719.
13. Kim AJ, Yong RJ, Urman RD. The role of transversus abdominis plane blocks in enhanced recovery after surgery pathways for open and laparoscopic colorectal surgery. J Laparoendosc Adv Surg Tech A. 2017; 27:909–914. PMID: 28742435.

14. Young J, Macpherson A, Thakerar A, Alexander M. Intrathecal morphine in postoperative analgesia for colorectal cancer surgery: a retrospective study. Pain Med. 2021; 22:402–406. PMID: 33164104.

15. Kong SK, Onsiong SM, Chiu WK, Li MK. Use of intrathecal morphine for postoperative pain relief after elective laparoscopic colorectal surgery. Anaesthesia. 2002; 57:1168–1173. PMID: 12437707.

16. Gehling M, Tryba M. Risks and side-effects of intrathecal morphine combined with spinal anaesthesia: a meta-analysis. Anaesthesia. 2009; 64:643–651. PMID: 19462494.

17. Sultan P, Halpern SH, Pushpanathan E, Patel S, Carvalho B. The effect of intrathecal morphine dose on outcomes after elective cesarean delivery: a meta-analysis. Anesth Analg. 2016; 123:154–164. PMID: 27089000.
18. DeSousa KA, Chandran R. Intrathecal morphine for postoperative analgesia: current trends. World J Anesthesiol. 2014; 3:191–202.

19. Fares KM, Mohamed SA, Abdel-Ghaffar HS. High dose intrathecal morphine for major abdominal cancer surgery: a prospective double-blind, dose-finding clinical study. Pain Physician. 2014; 17:255–264. PMID: 24850107.
20. Hutchins JL, Renfro L, Orza F, Honl C, Navare S, Berg AA. The addition of intrathecal morphine to a transversus abdominis plane block with liposome bupivacaine provides more effective analgesia than transversus abdominis plane block with liposome bupivacaine alone: a retrospective study. Local Reg Anesth. 2019; 12:7–13. PMID: 30863147.
21. Kunopart M, Chanthong P, Thongpolswat N, Intiyanaravut T, Pethuahong C. Effects of single shot femoral nerve block combined with intrathecal morphine for postoperative analgesia: a randomized, controlled, dose-ranging study after total knee arthroplasty. J Med Assoc Thai. 2014; 97:195–202. PMID: 24765899.
22. Huang YM, Lee YW, Huang YJ, Wei PL. Comparison of clinical outcomes between laparoscopic and open surgery for left-sided colon cancer: a nationwide populat ion-based study. Sci Rep. 2020; 10:75. PMID: 31919417.

23. Wongyingsinn M, Baldini G, Stein B, Charlebois P, Liberman S, Carli F. Spinal analgesia for laparoscopic colonic resection using an enhanced recovery after surgery programme: better analgesia, but no benefits on postoperative recovery: a randomized controlled trial. Br J Anaesth. 2012; 108:850–856. PMID: 22408272.

24. Ummenhofer WC, Arends RH, Shen DD, Bernards CM. Comparative spinal distribution and clearance kinetics of intrathecally administered morphine, fentanyl, alfentanil, and sufentanil. Anesthesiology. 2000; 92:739–753. PMID: 10719953.

25. Rodanant O, Sirichotewithayakorn P, Sriprajittichai P, Charuluxananan S. An optimal dose study of intrathecal morphine in gynecological patients. J Med Assoc Thai. 2003; 86 Suppl 2:S331–S337. PMID: 12930007.
26. Yu Y, Gao S, Yuen VM, Choi SW, Xu X. The analgesic efficacy of ultrasound-guided transversus abdominis plane (TAP) block combined with oral multimodal analgesia in comparison with oral multimodal analgesia after caesarean delivery: a randomized controlled trial. BMC Anesthesiol. 2021; 21:7. PMID: 33413104.

27. Tomaszewski D, Bałkota M, Truszczyński A, Machowicz A. Intrathecal morphine increases the incidence of urinary retention in orthopaedic patients under spinal anaesthesia. Anaesthesiol Intensive Ther. 2014; 46:29–33. PMID: 24643924.
28. Kuipers PW, Kamphuis ET, van Venrooij GE, van Roy JP, Ionescu TI, Knape JT, et al. Intrathecal opioids and lower urinary tract function: a urodynamic evaluation. Anesthesiology. 2004; 100:1497–1503. PMID: 15166570.
29. Wiener JG, Gunnells D, Wood L, Chu DI, Cannon J, Kennedy GD, et al. Early removal of catheters in an Enhanced Recovery Pathway (ERP) with intrathecal opioid inject ion does not a f fect postoperative urinary outcomes. Am J Surg. 2020; 219:983–987. PMID: 31590888.
SUPPLEMENTARY MATERIALS
Supplementary Fig. 1 and Supplementary Table 1 can be found via https://doi.org/10.4174/astr.2021.101.4.221.
Supplementary Table 1
Clinical outcome differences between the intrathecal morphine with or without surgical transverse abdominal block without anastomosis leakage
Supplementary Fig. 1
Differences of side effects in the intrathecal morphine according to sex.
Fig. 1
CONSORT (consolidated standards of reporting trials) flow diagram. ITA, intrathecal analgesia; TAPB, transverse abdominis plane block.
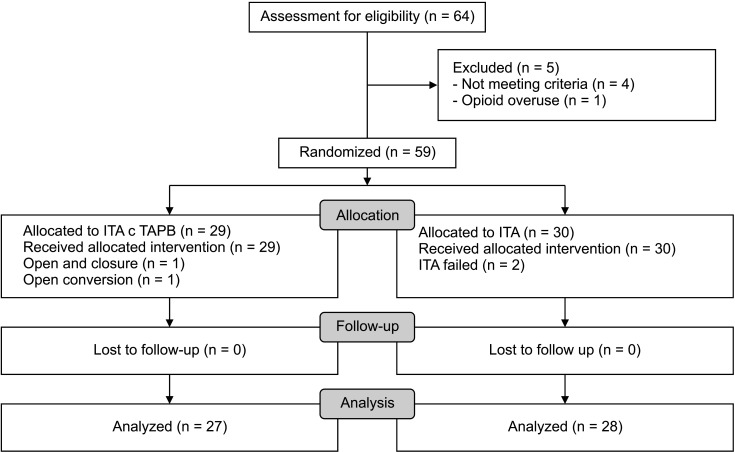
Fig. 2
Comparison of opioid consumption and pain scores between intrathecal analgesia (ITA) patients with or without transverse abdominis plane block (TAPB) at postoperative 0 and 24 hours. (A) Opioid at 0 hour, (B) pain score at 0 hour, (C) opioid at 24 hours, and (D) pain score at 24 hour.
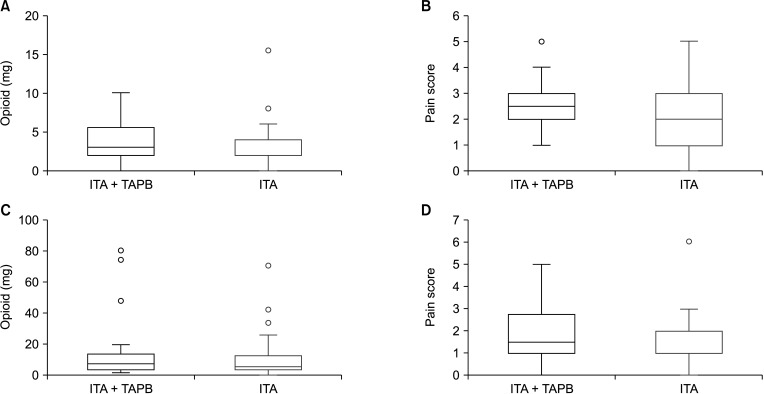
Table 1
Baseline characteristics and details by technique
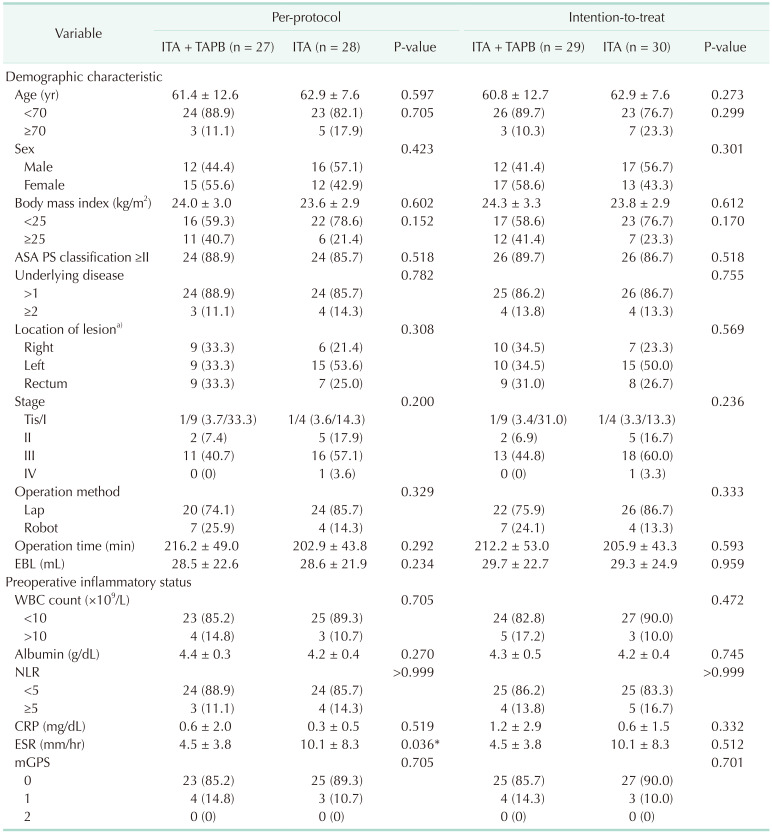
Values are presented as mean ± standard deviation or number (%).
ITA, intrathecal analgesia; TAPB, transverse abdominis plane block; ASA, American Society of Anesthesiologists; PS, physical status; EBL, estimated blood loss; NLR, neutrophil to lymphocyte ratio; mGPS, modified Glasgow prognostic score.
a)Diverticulum, huge adenoma, desmoid tumor, appendiceal cancer, and colorectal cancer.
*Statistically significant (P < 0.05).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download