1. de Oliveira JD, Carvalho LS, Gomes AM, Queiroz LR, Magalhães BS, Parachin NS. Genetic basis for hyper production of hyaluronic acid in natural and engineered microorganisms. Microb Cell Fact. 2016; 15:119. PMID:
27370777.

2. Kogan G, Soltés L, Stern R, Gemeiner P. Hyaluronic acid: a natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol Lett. 2007; 29:17–25. PMID:
17091377.

3. Cai Z, Zhang H, Wei Y, Cong F. Hyaluronan-inorganic nanohybrid materials for biomedical applications. Biomacromolecules. 2017; 18:1677–1696. PMID:
28485601.

4. Voigt J, Driver VR. Hyaluronic acid derivatives and their healing effect on burns, epithelial surgical wounds, and chronic wounds: a systematic review and meta-analysis of randomized controlled trials. Wound Repair Regen. 2012; 20:317–331. PMID:
22564227.

5. Neuman MG, Nanau RM, Oruña-Sanchez L, Coto G. Hyaluronic acid and wound healing. J Pharm Pharm Sci. 2015; 18:53–60. PMID:
25877441.

6. Price RD, Myers S, Leigh IM, Navsaria HA. The role of hyaluronic acid in wound healing: assessment of clinical evidence. Am J Clin Dermatol. 2005; 6:393–402. PMID:
16343027.
7. Mansouri Y, Goldenberg G. Update on hyaluronic acid fillers for facial rejuvenation. Cutis. 2015; 96:85–88. PMID:
26367746.
8. Johanson JF, Sonnenberg A. The prevalence of hemorrhoids and chronic constipation: an epidemiologic study. Gastroenterology. 1990; 98:380–386. PMID:
2295392.
9. National Health Insurance Service. National Health Insurance Statistical Yearbook in Korea, 2018. Wonju (KR): National Health Insurance Service;2018.
10. Jacobs D. Clinical practice. Hemorrhoids. N Engl J Med. 2014; 371:944–951. PMID:
25184866.
11. Nicholson TJ, Armstrong D. Topical metronidazole (10 percent) decreases posthemor rhoidectomy pain and improves healing. Dis Colon Rectum. 2004; 47:711–716. PMID:
15054681.
12. Gupta PJ, Heda PS, Kalaskar S, Tamaskar VP. Topical sucralfate decreases pain after hemorrhoidectomy and improves healing: a randomized, blinded, controlled study. Dis Colon Rectum. 2008; 51:231–234. PMID:
18095028.

13. Karanlik H, Akturk R, Camlica H, Asoglu O. The effect of glyceryl trinitrate ointment on posthemorrhoidectomy pain and wound healing: results of a randomized, double-blind, placebo-controlled study. Dis Colon Rectum. 2009; 52:280–285. PMID:
19279424.
14. Eshghi F, Hosseinimehr SJ, Rahmani N, Khademloo M, Norozi MS, Hojati O. Effects of A loe vera cream on posthemor rhoidectomy pain and wound healing: results of a randomized, blind, placebo-control study. J Altern Complement Med. 2010; 16:647–650. PMID:
20569031.
15. Xu X, Li X, Zhang L, Liu Z, Pan Y, Chen D, et al. Enhancement of wound healing by the traditional Chinese medicine herbal mixture sophora flavescens in a rat model of perianal ulceration. In Vivo. 2017; 31:543–549. PMID:
28652418.
16. Ala S, Alvandipour M, Saeedi M, Hamidian M, Shiva A, Rahmani N, Faramarzi F. Effects of topical atorvastatin (2 %) on posthemorrhoidectomy pain and wound healing: a randomized double-blind placebo-controlled clinical trial. World J Surg. 2017; 41:596–602. PMID:
27738832.
17. Duman N, Duman R, Tosun M, Akıcı M, Göksel E, Gökçe B, et al. Topical folinic acid enhances wound healing in rat model. Adv Med Sci. 2018; 63:347–352. PMID:
30092503.

18. Chen S, Huan Z, Zhang L, Chang J. The clinical application of a silicate-based wound dressing (DermFactor®) for wound healing after anal surgery: a randomized study. Int J Surg. 2018; 52:229–232. PMID:
29481992.

19. DK KweonSH Park. Jinwoo Bio Co. Ltd.Method for manufacturing hyaluronate fibers by using melt spinning and hyaluronate fibers manufactured thereby. United States patent. US 20180243471A1. 2018. 8. 30.
20. Zhou X, Patel D, Sen S, Shanmugam V, Sidawy A, Mishra L, et al. Poly-ADP-ribose polymerase inhibition enhances ischemic and diabetic wound healing by promoting angiogenesis. J Vasc Surg. 2017; 65:1161–1169. PMID:
27288104.

21. Gallagher KA, Joshi A, Carson WF, Schaller M, Allen R, Mukerjee S, et al. Epigenetic changes in bone marrow progenitor cells influence the inflammatory phenotype and alter wound healing in type 2 diabetes. Diabetes. 2015; 64:1420–1430. PMID:
25368099.

22. Aya KL, Stern R. Hyaluronan in wound healing: rediscovering a major player. Wound Repair Regen. 2014; 22:579–593. PMID:
25039417.

23. Hoffmann A, Hoing JL, Newman M, Simman R. Role of hyaluronic acid treatment in the prevention of keloid scarring. J Am Coll Clin Wound Spec. 2013; 4:23–31. PMID:
24936445.
24. Watson AJ, Cook J, Hudson J, Kilonzo M, Wood J, Bruhn H, et al. A pragmatic multicentre randomised controlled trial comparing stapled haemorrhoidopexy with traditional excisional surgery for haemorrhoidal disease: the eTHoS study. Health Technol Assess. 2017; 21:1–224.

25. Liu JW, Lin CC, Kiu KT, Wang CY, Tam KW. Effect of glyceryl trinitrate ointment on pain control after hemorrhoidectomy: a meta-analysis of randomized controlled trials. World J Surg. 2016; 40:215–224. PMID:
26578318.

26. MottT , Latimer K, Edwards C. Hemorrhoids: diagnosis and treatment options. Am Fam Physician. 2018; 97:172–179. PMID:
29431977.
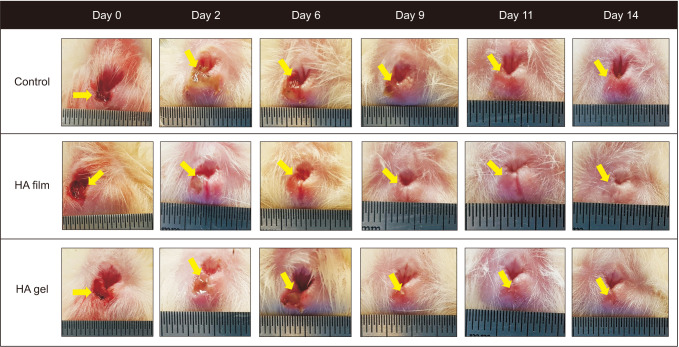
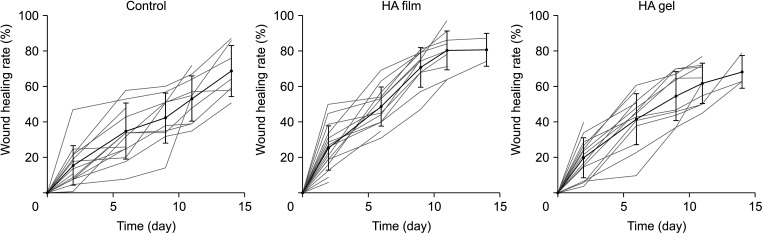




 PDF
PDF Citation
Citation Print
Print



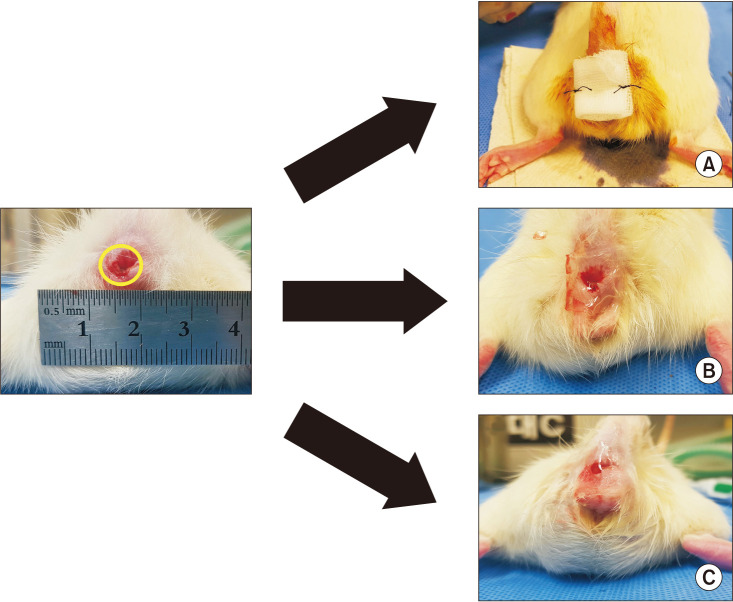
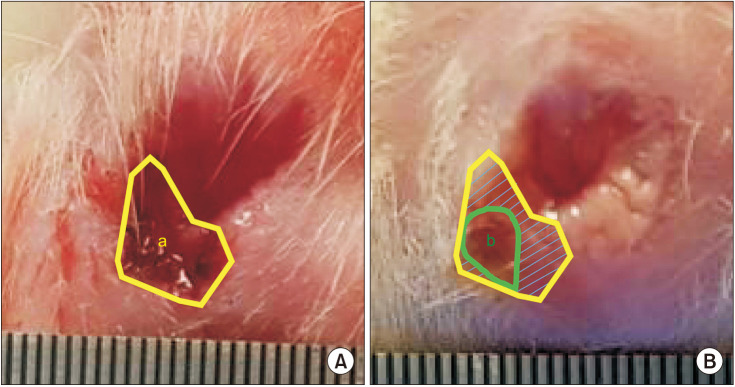
 XML Download
XML Download