Abstract
Purpose
We aimed to investigate whether adjuvant oxaliplatin-based chemotherapy after treatment for hepatic metastasis affects recurrence or survival and to determine the risk factors for recurrence or survival.
Methods
Forty-six patients who underwent curative treatment for hepatic metastasis from colorectal cancer between July 2009 and December 2017 were included from a retrospectively collected patient database. Curative resection included hepatic resection, radiofrequency ablation (RFA), or a combination of both, followed by adjuvant chemotherapy with oxaliplatin-based chemotherapy.
Results
Thirty-seven patients (80.4%) had colon cancer and 9 (19.6%) had rectal cancer. Twenty-six patients (56.5%) underwent hepatic resection, 7 (15.2%) RFA, and 13 (28.3%) hepatic resection and RFA. Thirty-two patients (69.6%) underwent chemotherapy after hepatic treatment. The recurrence incidence was 50% in the non-chemotherapy group and 46.9% in the chemotherapy group (P > 0.999). The incidence of death was 7.1% in the non-chemotherapy group and 18.8% in the chemotherapy group (P = 0.657). The recurrence risk factors were N stage (N0 vs. N2; P = 0.013, P = 0.005) and bilobed hepatic metastasis (P = 0.027, P = 0.009) in the univariate and multivariate analyses, respectively. However, chemotherapy after hepatic treatment was not a risk factor for disease-free survival (DFS) or overall survival (OS) in the univariate and multivariate analyses (P = 0.656 and P = 0.414, respectively; P = 0.510 and P = 0.459, respectively).
Go to : 
Colorectal cancer (CRC) is the third most common cancer worldwide, accounting for 10% of all cancers. It is a significant cause of morbidity and the second most common cause of cancer-related mortality [1]. Extensive epidemiological studies from multiple European centers have demonstrated that the incidence of both synchronous and metachronous hepatic metastases in patients with CRC is approximately 25%. A quarter of patients with hepatic metastases were treated with curative intent [2345]. Although hepatic resection is a curative treatment, more than half of patients undergoing hepatic resection for colorectal cancer hepatic metastasis (CRCHM) develop recurrence [6]. Thus, the National Comprehensive Cancer Network treatment guidelines recommend adjuvant chemotherapy for CRCHM after hepatic resection or local therapy such as radiofrequency ablation (RFA). However, several studies have shown no significant difference in the overall survival (OS) of patients who underwent perioperative chemotherapy and surgery alone [789]. Particularly, for many adjuvant chemotherapy studies, it has been challenging to determine whether there are benefits in performing adjuvant chemotherapy after hepatic resection because of the small sizes of the studies and the types of clinicopathological variables collected. Therefore, we investigated whether adjuvant oxaliplatin-based chemotherapy after treatment for hepatic metastasis affected recurrence or survival. In addition to chemotherapy, we investigated the presence of other risk factors affecting disease-free survival (DFS) or OS.
Go to : 
This study was approved by the Institutional Review Board of Konkuk University School of Medicine (No. KUMC 2021-03-053) with a waiver for informed consent. We retrospectively included 80 patients who underwent curative treatment for hepatic metastasis from CRC between July 2009 and December 2017 in the current study. Curative treatment included hepatic resection, RFA, or a combination of both. Postoperative adjuvant chemotherapy included oxaliplatin-based FOLFOX4 and mFOLFOX6. The exclusion criteria were as follows: patients who had extrahepatic lesions before surgery for hepatic metastasis and had undergone neoadjuvant chemotherapy, a cycle of adjuvant chemotherapy less than 6 times, or CRC surgery at other hospitals. If recurrence occurred within 6 months after treatment for hepatic metastasis, extrahepatic lesions or the remnant hepatic area were considered. Therefore, these patients were also excluded. Finally, 46 patients were included in this study.
We analyzed the medical data of all the eligible patients. The parameters analyzed included age, sex, and the primary cancer lesions of each patient. The colon was defined from the cecum to the rectosigmoid colon. The rectum was defined from the lower border of the rectosigmoid junction to the dentate line. The types of hepatic metastases were categorized into synchronous and metachronous types. Synchronous cancers were defined as hepatic metastasis within 6 months of the first primary CRC, while metachronous cancers were defined as hepatic metastasis occurring more than 6 months later. The date of treatment for CRC or CRCHM was based on the date of surgery, and the time of recurrence was based on the date of the formal reading of the imaging study.
For all 46 patients, we have provided information on the oxaliplatin-based chemotherapy effect after treatment of hepatic metastasis based on the data published at that time. Of these, chemotherapy was performed only in the patients who opted to receive it. If a poor performance was expected or the patient refused, it was not administered.
The mFOLFOX6 regimen consisted of an intravenous injection of oxaliplatin 85 mg/m2, folinic acid 200 mg/m2 (over 2 hours), and fluorouracil 400 mg/m2 (bolus) and 2,400 mg/m2 (continuous 4–6 hours infusion). The FOLFOX4 regimen consisted of an intravenous injection of oxaliplatin 85 mg/m2, folinic acid 200 mg/m2 (over 2 hours), as well as fluorouracil 400 mg/m2 (bolus) and 600 mg/m2 (continuous 22 hours infusion) on day 1, followed by folinic acid 200 mg/m2 (over 2 hours), and fluorouracil 400 mg/m2 (bolus) and 600 mg/m2 (continuous22 hours infusion) on day 2. Each chemotherapy cycle lasted 14 days, with the subsequent cycle starting on day 15. The response was assessed every 3 cycles.
According to the type of variable and their distribution, the groups were compared using the Student t-test and chi-square test for qualitative variables. The Cox model was used to identify the significant factors associated with DFS and OS using univariate and multivariate analyses. Statistical significance was considered to be associated with a P-value less than 0.05.
Go to : 
Patient characteristics are shown in Table 1. Of the 46 patients, 33 were males and 13 were females, with a mean age of 62 years (range, 35–80 years). Thirty-seven patients (80.4%) had colon cancer, and 9 (19.6%) had rectal cancer. Twenty-six patients (56.5%) underwent hepatic resection, 7 (15.2%) underwent RFA, and 13 (28.3%) underwent both hepatic resection and RFA. Thirteen patients (28.3%) had metachronous hepatic metastasis, and 33 patients (71.7%) had synchronous hepatic metastasis. Thirty-two patients (69.6%) underwent chemotherapy after hepatic treatment, and 14 patients (30.4%) did not receive chemotherapy. Recurrence was observed in 22 patients (47.8%), and 7 patients (15.2%) died. The mean interval period of hepatic treatment was 5.9 months (range, 0–51 months) and the mean follow-up from the time of the hepatic treatment was 63.8 months (range, 15–117 months). The mean total follow-up period after surgery for CRC was 69.7 months (range, 15–121 months).
We investigated whether there were clinical differences between the groups who received chemotherapy (n = 32) and those who did not (n = 14) (Table 2). Patients with synchronous hepatic metastasis tended to have a higher chemotherapy rate than those with metachronous metastasis (P = 0.001). More patients in the synchronous group underwent surgical resection (P = 0.038). There was no significant difference in recurrence (P > 0.999) or death (P = 0.413) between those who received chemotherapy and those who did not.
The 46 patients were classified according to those with recurrence (n = 22) or those without (n = 24), and the N stage was significantly different (P = 0.022) in the recurrence group (Table 3).
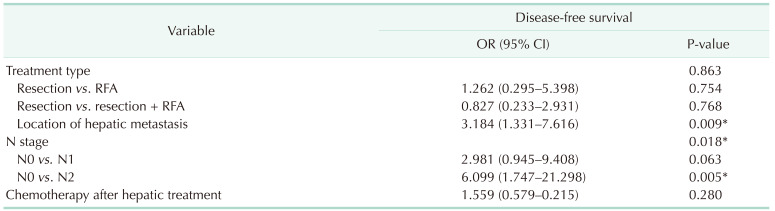
In the univariate analysis, treatment type (resection vs resection plus RFA, P = 0.044), location of hepatic metastasis (unilobed vs. bilobed, P = 0.027), and N stage (N0 vs. N2, P = 0.013) were associated with worse DFS. Chemotherapy after hepatic treatment was not associated with either DFS or OS (P = 0.656, P = 0.414) (Table 4).
In the multivariate analysis, location of hepatic metastasis (unilobed vs. bilobed, P = 0.009) and N stage (N0 vs. N2, P = 0.005) were associated with DFS (Table 5).
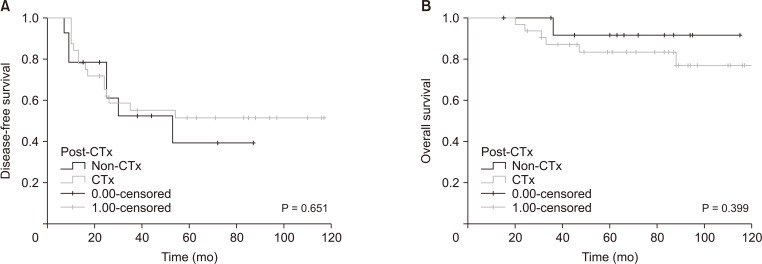
Fig. 1 shows the OS for posttreatment chemotherapy in patients with hepatic metastasis of CRC. There was no significant difference in OS between patients who received chemotherapy and those who did not receive chemotherapy (P = 0.399).
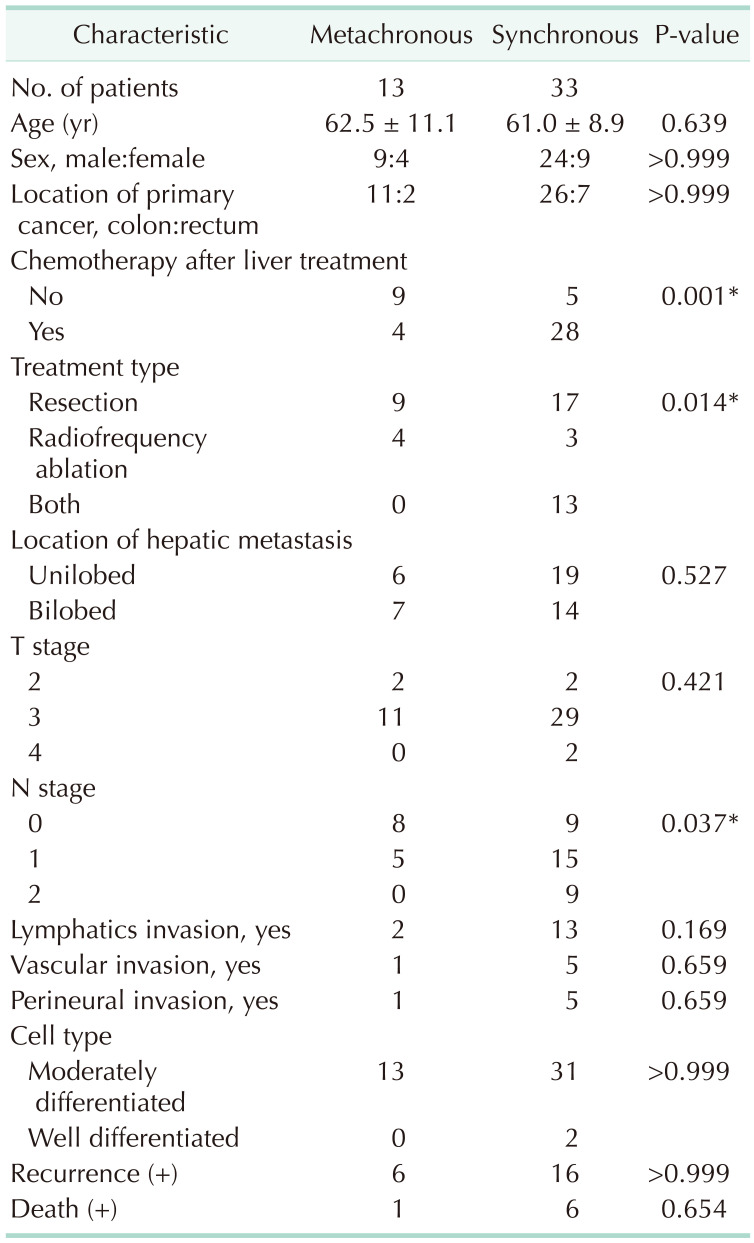
We investigated whether there were clinical differences according to the type of hepatic metastasis (metachronous, 13 patients; synchronous, 33 patients). The synchronous patient group received more chemotherapy (28 of 33, 84.8%) than the metachronous group (4 of 13, 30.8%) (P = 0.001). Hepatic resections were performed more frequently in the synchronous group than in the metachronous group (30 of 33, 90.9% vs. 3 of 33, 9.1%; P = 0.014). There was no significant difference in recurrence (P > 0.999) or death (P = 0.654) between the metachronous and synchronous groups (Table 6).
Go to : 
The most common site for tumor recurrence is the residual liver, which is involved in 45%–75% of recurrence cases in CRCHM. In most cases, chemotherapy for CRCHM has been established and studied as an adjuvant therapy because the liver is the solitary site of the first recurrence in up to 40% of all CRC patients [10].
Adjuvant chemotherapy is known to reduce recurrence in patients with CRCHM after hepatic resection [11]. The EORTC 40983 trial showed that perioperative chemotherapy with FOLFOX4 increased progression-free survival (PFS) compared with surgery alone in patients with an initially resectable hepatic metastasis from CRC, but there was no observed difference in OS [7]. Similarly, a study by Portier et al. [12] also reported that perioperative therapy with adjuvant fluorouracil and folinic acid after hepatic resection increased PFS. However, Kanemitsu et al. [8] recently reported that postoperative chemotherapy with mFOLFOX6 improved DFS, but worsened OS, compared to surgery alone. Thus, adjuvant mFOLFOX6 does not benefit OS after hepatectomy. Most trials also investigated DFS [91213], but there was a lack of randomization in many studies, which makes the results difficult to interpret [14] and prevents the conclusion that chemotherapy is helpful after hepatic metastasis surgery.
Sometimes, target agents are added as first-line chemotherapy after resection of hepatic metastasis in clinical practice. Although there was no increase in perioperative morbidity and mortality, bevacizumab did not affect patient outcomes [15]. In another study, FOLFOX with cetuximab after hepatic resection was associated with shorter PFS because of the higher complication rates [16].
In the present study, we investigated the effect of oxaliplatin-based chemotherapy after hepatic treatment and found that there was no difference in DFS and OS between the group that received chemotherapy after hepatic treatment and the group that only received hepatic treatment. In addition, hepatic resection is the best curative strategy; however, if resection is not possible, RFA can be considered [17]. In this study, the treatment of hepatic metastasis included not only resection but also RFA [18]. This may therefore be a limitation of the study, in addition to the small number of patients. There were no risk factors affecting OS, and the recurrence rate was higher in the group that underwent hepatic resection and RFA than in the group that underwent resection only. This is because resection and RFA may be performed in cases with bilobed metastasis than those with in unilobed metastasis. This is consistent with the fact that patients with bilobed metastasis have a higher recurrence rate than those with unilobed metastasis (Tables 4, 5).
Meanwhile, patients with synchronous CRCHM tended to have a higher chemotherapy rate than those with metachronous disease (P = 0.001). Several studies have found that the survival of synchronous CRCHM patients is significantly worse than that of metachronous patients [192021]. Although studies differ in how they define the interval for synchronous versus metachronous CRCHM, it is generally agreed that synchronous disease represents more aggressive tumor biology with a heightened risk of disease recurrence and reduced patient survival than metachronous disease [22]. These data suggest that chemotherapy is most advantageous for patients with more aggressive tumor biology (synchronous or early metachronous CRCHM) [23]. In the case of the synchronous group, adjuvant chemotherapy was administered for primary CRC, and the low percentage of chemotherapy in the metachronous group was because chemotherapy for primary CRC had already been completed, and thus additional chemotherapy was not performed after hepatic metastasis treatment. Nevertheless, there was no significant difference in recurrence or death between the metachronous and synchronous groups in this study.
This study has several limitations. Since this study was a retrospective analysis, there was a selective bias. The small sample size was also a limitation, and we could not compare the OS. Therefore, further investigation with a larger number of cases is required.
In conclusion, we found that oxaliplatin-based chemotherapy after hepatic treatment did not affect DFS or OS. Thus, each decision on postoperative chemotherapy should be made by taking into consideration the prior therapy, treatment type, location of hepatic metastasis, and N stage.
Go to : 
References
1. Dekker E, Tanis PJ, Vleugels JL, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019; 394:1467–1480. PMID: 31631858.

2. Engstrand J, Nilsson H, Strömberg C, Jonas E, Freedman J. Colorectal cancer liver metastases: a population-based study on incidence, management and survival. BMC Cancer. 2018; 18:78. PMID: 29334918.

3. van der Geest LG, Lam-Boer J, Koopman M, Verhoef C, Elferink MA, de Wilt JH. Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin Exp Metastasis. 2015; 32:457–465. PMID: 25899064.

4. Hackl C, Neumann P, Gerken M, Loss M, Klinkhammer-Schalke M, Schlitt HJ. Treatment of colorectal liver metastases in Germany: a ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer. 2014; 14:810. PMID: 25369977.

5. Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006; 244:254–259. PMID: 16858188.

6. Angelsen JH, Viste A, Løes IM, Eide GE, Hoem D, Sorbye H, et al. Predictive factors for time to recurrence, treatment and post-recurrence survival in patients with init ial ly resected colorectal liver metastases. World J Surg Oncol. 2015; 13:328. PMID: 26631156.

7. Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013; 14:1208–1215. PMID: 24120480.

8. Kanemitsu Y, Kato T, Shimizu Y, Inaba Y, Shimada Y, Nakamura K, et al. A randomized phase II/III trial comparing hepatectomy followed by mFOLFOX6 with hepatectomy alone as treatment for liver metastasis from colorectal cancer: Japan Clinical Oncology Group Study JCOG0603. Jpn J Clin Oncol. 2009; 39:406–409. PMID: 19389795.

9. Kemeny MM, Adak S, Gray B, Macdonald JS, Smith T, Lipsitz S, et al. Combined-modalit y treatment for resectable metastatic colorectal carcinoma to the liver: surgical resection of hepatic metastases in combination with continuous infusion of chemotherapy: an intergroup study. J Clin Oncol. 2002; 20:1499–1505. PMID: 11896097.
10. Parks R, Gonen M, Kemeny N, Jarnagin W, D'Angelica M, DeMatteo R, et al. Adjuvant chemotherapy improves survival after resection of hepatic colorectal metastases: analysis of data from two continents. J Am Coll Surg. 2007; 204:753–763. PMID: 17481478.

11. Petrelli NJ. Perioperative or adjuvant therapy for resectable colorectal hepatic metastases. J Clin Oncol. 2008; 26:4862–4863. PMID: 18794535.

12. Portier G, Elias D, Bouche O, Rougier P, Bosset JF, Saric J, et al. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol. 2006; 24:4976–4982. PMID: 17075115.

13. Kemeny N, Huang Y, Cohen AM, Shi W, Conti JA, Brennan MF, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med. 1999; 341:2039–2048. PMID: 10615075.

14. Kemeny N, Capanu M, D'Angelica M, Jarnagin W, Haviland D, Dematteo R, et al. Phase I trial of adjuvant hepatic arterial infusion (HAI) with floxuridine (FUDR) and dexamethasone plus systemic oxaliplatin, 5-fluorouracil and leucovorin in patients with resected liver metastases from colorectal cancer. Ann Oncol. 2009; 20:1236–1241. PMID: 19233901.

15. Chaudhury P, Hassanain M, Bouganim N, Salman A, Kavan P, Metrakos P. Perioperat ive chemotherapy with bevacizumab and liver resection for colorectal cancer liver metastasis. HPB (Oxford). 2010; 12:37–42. PMID: 20495643.
16. Bridgewater JA, Pugh SA, Maishman T, Eminton Z, Mellor J, Whitehead A, et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis (New EPOC): long-term results of a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2020; 21:398–411. PMID: 32014119.
17. Mima K, Beppu T, Chikamoto A, Miyamoto Y, Nakagawa S, Kuroki H, et al. Hepatic resection combined with radiofrequency ablation for initially unresectable colorectal liver metastases after effective chemotherapy is a safe procedure with a low incidence of local recurrence. Int J Clin Oncol. 2013; 18:847–855. PMID: 22940848.

18. Kim YI, Park IJ, Kim JE, Kim SY, Park JH, Lee JH, et al. Hepatic resection after neoadjuvant chemotherapy for patients with liver metastases from colorectal cancer: need for cautious planning. Ann Surg Treat Res. 2019; 97:245–253. PMID: 31742209.

19. Neo EL, Beeke C, Price T, Maddern G, Karapetis C, Luke C, et al. South Australian clinical registry for metastatic colorectal cancer. ANZ J Surg. 2011; 81:352–357. PMID: 21518185.

20. van der Pool AE, Lalmahomed ZS, Ozbay Y, de Wilt JH, Eggermont AM, Jzermans JN, et al. ‘Staged’ liver resection in synchronous and metachronous colorectal hepatic metastases: differences in clinicopathological features and outcome. Colorectal Dis. 2010; 12(10 Online):e229–e235. PMID: 19912286.

21. Ng WW, Cheung YS, Wong J, Lee KF, Lai PB. A preliminary analysis of combined liver resection with new chemotherapy for synchronous and metachronous colorectal liver metastasis. Asian J Surg. 2009; 32:189–197. PMID: 19892621.

22. Adam R, de Gramont A, Figueras J, Kokudo N, Kunstlinger F, Loyer E, et al. Managing synchronous liver metastases from colorectal cancer: a multidisciplinary international consensus. Cancer Treat Rev. 2015; 41:729–741. PMID: 26417845.

23. Wisneski AD, Jin C, Huang CY, Warren R, Hirose K, Nakakura EK, et al. Synchronous versus metachronous colorectal liver metastasis yields similar survival in modern era. J Surg Res. 2020; 256:476–485. PMID: 32798995.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download