INTRODUCTION
Recently, advanced stage of gastric cancer has decreased in great number by early cancer detection of biennial routine endoscopy in the national cancer screening program in Korea [
123]. Moreover, perforated gastric cancer, which mostly develops in advanced and untreated states, is a rare condition in clinical situation [
4]. Despite its rarity, diffuse peritonitis from perforation allows only limited time for sufficient evaluation of the disease, so surgeons should be aware of this condition in diagnosis and management [
5]. Even worse for diagnosis, intraoperative frozen section or endoscopy is often unavailable in night-time operation.
A balanced surgical strategy should be selected in perforated gastric cancer, considering both peritonitis and invasiveness of malignancy. In the past, 1-stage gastrectomy was usually performed for suspected gastric cancer perforation cases. However, this strategy has the risk of surgery done under an uncertain diagnosis for gastric cancer or not. Introduction of 2-stage surgery, which includes immediate treatment on acute peritonitis with closure of the perforation site followed by curative gastrectomy with adequate lymphadenectomy, has proven to improve oncological outcomes over conventional 1-stage surgery [
6789]. Diagnosis and evaluation for the cancer status is possible during the interval of 2-stage.
Although the introduction of laparoscopy brought definite benefits in surgical outcomes and comparable oncological outcomes in gastric cancer, the feasibility of laparoscopic approach in perforated gastric cancer has not been analyzed yet and is still controversy [
9101112]. Therefore, this study was aimed to explore the general characteristics of gastric cancer perforation and assess the safety and feasibility of laparoscopic 2-stage surgery in the treatment of perforated gastric cancer.
Go to :

METHODS
Patients and data collection
Out of 2,318 patients with gastric cancer in Yeouido St. Mary's Hospital from January 1990 to December 2017, 22 patients (0.9%) with perforated gastric cancer were retrospectively evaluated with medical records. We excluded 2 patients with distant organ metastasis who underwent only palliative surgery, and enrolled 20 patients in stage I, II, and III with curative gastrectomy in our study. Emergent surgery was performed in all patients and decision for surgical strategy, whether to perform 1-stage or 2-stage procedure, was determined by a single surgeon. Out of 20 patients, 15 patients (75.0%) underwent 1-stage surgery and 5 patients (25.0%) with 2-stage surgery.
The study was performed in accordance with the ethical guidelines of the World Medical Association Declaration of Helsinki 2013. This study was approved by the Institutional Review Board of the Ethics Committee of the College of Medicine, The Catholic University of Korea (No. SC19RESI0114) and informed consent was wavied.
Surgical strategy and procedure of 2-stage gastrectomy
When the disease was clinically confirmed, decision of surgical management on perforated gastric cancer was made by a single surgeon. In actual, general strategy was changed from 2009 when the surgeon performed laparoscopic surgery more friendly. Before 2009, 1-stage open laparotomy and lymph node dissection (LND) was performed. From 2009, all the cases were initially started with laparoscopic exploration.
In the first surgery, massive irrigation, and primary closure with or without Foley catheter tube gastrostomy were done on purpose of inflammation control surgery. In the case of primary closure only, omentopexy was added at the primary closure site. In the case of large perforation site with higher risk of further leakage with only primary closure, Foley catheter as tube gastrostomy was inserted (
Fig. 1). This tube gastrostomy was kept until the second surgery.
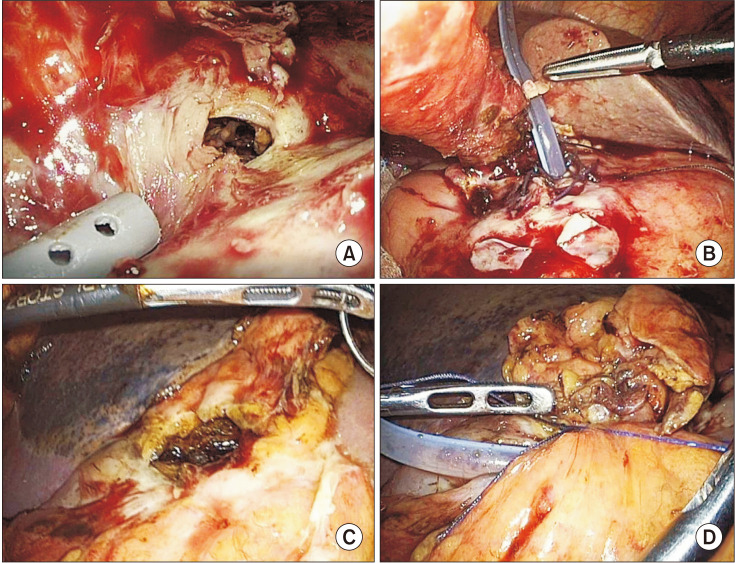 | Fig. 1First step surgery of laparoscopic primary closure with Foley catheter insertion as tube gastrostomy. (A, C) Large perforation site with suspicious serosal invasion of gastric cancer. (B, D) Tube gastrostomy with Foley catheter insertion through perforation site.
|
After the first surgery, proper evaluation on the extent of gastric cancer was made for planning the second step surgery. Preoperative endoscopy was safely performed to confirm the cancer pathology through biopsy, and to check the exact location of the lesion and the status of tube gastrostomy (
Fig. 2). Proper reading of abdominal CT by a radiologist and additional radiologic evaluation was done to estimate the depth of tumor, local invasiveness, and distant metastasis. In the second surgery, laparoscopic approach was first attempted on the planned schedule, and adequate lymphadenectomy was done depending on the clinical stage of gastric cancer.
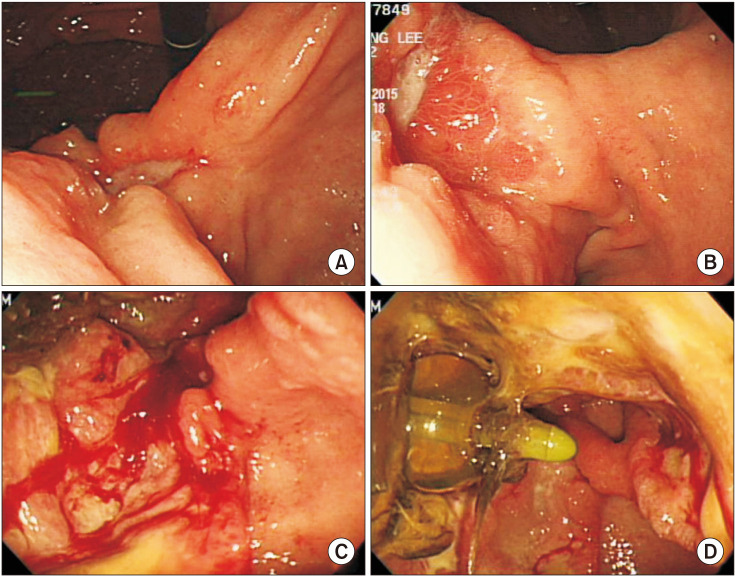 | Fig. 2Preoperative endoscopy after the first surgery. (A–C) Ulcerofungating lesion which is pathologically confirmed with gastric cancer. (D) Foley catheter tube gastrostomy insertion state.
|
Study design
Whole perforated gastric cancer patients were included to describe the general characteristic of perforated gastric cancer. The patients were divided into 2 groups depending on surgical strategy, 1-stage gastrectomy or 2-stage gastrectomy. We summarized clinicopathologic features and surgical outcomes according to 2 groups to overview the general characteristics. Pathological stage was classified according to the general rules of the American Joint Committee on Cancer 8th edition of TNM staging. For surgical outcomes, the type and method of surgery, degree of LND, and retrieved lymph node (LN) were evaluated. Detailed information was described regarding the 2-stage gastrectomies including time interval between the first and second surgeries, result of preoperative endoscopic biopsy, presence of Foley catheter as tube gastrostomy in the first surgery, and postoperative complications. At last, survival analysis was done.
Statistical analysis
Since the number of patients enrolled in this study is relatively small and normal distribution is not formed, continuous variables were compared using the non-parametric Mann-Whitney test. Categorical variables of the groups were compared using the chi-square test, and if the expected value less than 5 is more than 20% of the total, the Fisher exact test was used. Overall survival and disease-free survival rates were calculated using the Kaplan-Meier method, and survival curves were compared among the groups using the log-rank test. P-values under 0.05 were considered to indicate statistical significance. All statistical analyses were performed using IBM SPSS Statistics for Windows ver. 24.0 (IBM Corp., Armonk, NY, USA).
Go to :

RESULTS
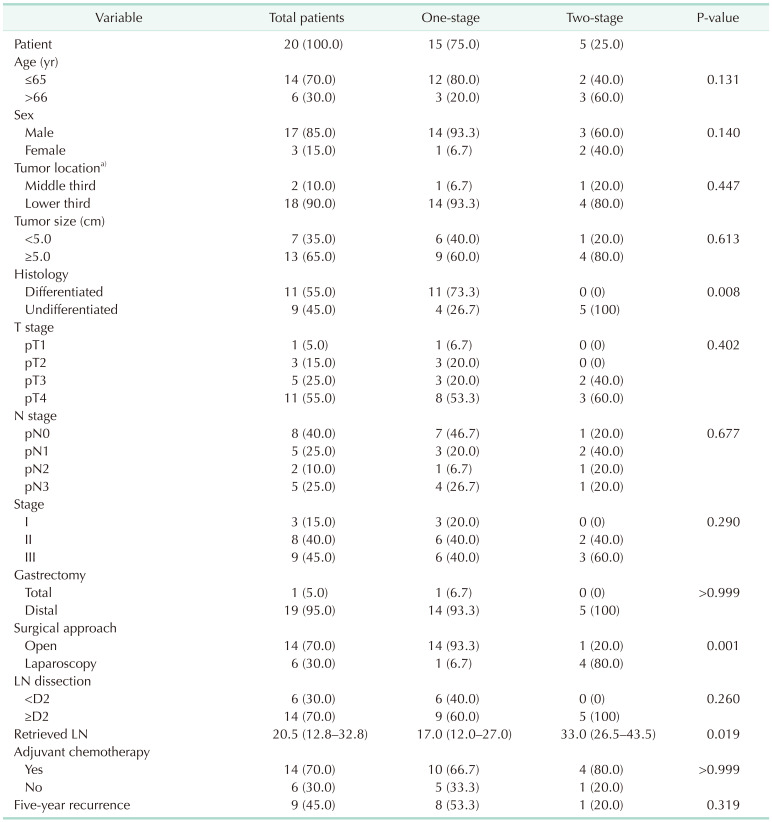
The clinicopathological features and surgical outcomes of perforated gastric cancer were compared in 1-stage and 2-stage gastrectomy groups and summarized in
Table 1. Median follow-up duration was 38 months. The mean age of patients was 57.5 years, and the male-female ratio was 5.7:1. Despite the perforation of cancer, nonserosal invasion (pathologic [p] T1, pT2, and pT3) was shown in 9 patients (45.0%) and noninvasion (pN0) of LN was found in 8 patients (40.0%), resulting in 3 patients (15.0%) with stage I and 8 patients (40.0%) in stage II. Regarding the LND, D2 extended LND was performed in 14 patients (70.0%), whereas other 6 patients did not undergo D2 LND due to severe peritonitis. Among those 6 limited LND patients, 5 patients yielded less than 15 LNs at the final pathologic report. All patients of 2-stage gastrectomy underwent D2 or more than D2 LND and yielded more than 15 LNs. Total 9 patients (45.0%) had recurrence; 5 patients with peritoneal seeding, 2 patients in remnant stomach, 1 patient in liver, and 1 patient in celiac axis LN.
Table 1
Clinicopathological features and surgical outcomes of perforated gastric cancer

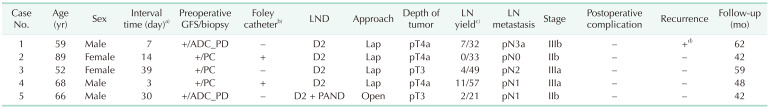
Detailed analysis of surgical outcomes in 2-stage gastrectomy group was evaluated in
Table 2. In the first step surgery, 2 patients had a comparatively large perforation site and a Foley catheter was introduced through the perforation site and as gastrostomy to prevent secondary leakage, as shown in
Fig. 1. No secondary leakage or postoperative complication after first step surgery was found in all patients. The timing of second step surgeries was determined according to patients' condition and surgeon's decision. Those were done within 3 weeks (3, 7, and 14 days) in 3 patients, and after 4 weeks (30 and 39 days) in 2 patients. Before curative gastrectomy, all the patients underwent diagnostic gastroscopy and emergency histologic confirmation was done as shown in
Fig. 2. In second step surgery, except for 1 patient for D2 plus paraaortic LND, 4 patients underwent laparoscopic D2 LND. Patients with 2-stage gastrectomy were performed with significantly higher rate of laparoscopic approach (6.7%
vs. 80.0%, P = 0.001), higher rate of D2 LND (60.0%
vs. 100.0%, P = 0.260) and significant higher number of retrieved LN (median [range]: 17.0 [12.0–27.0]
vs. 33.0 [26.5–43.5], P = 0.019).
Table 2
Surgical outcomes of laparoscopic 2-stage gastrectomy in perforated gastric cancer

In surgical findings of the second operation, severe adhesions were found among omentum, liver, and gastric perforation site. However, there was no severe adhesion at the secondtier LN dissection area, so sufficient D2 LND was performed without technical difficulty (
Fig. 3). Significantly more LNs were retrieved in 2-stage gastrectomy group compared to 1-step surgery (median [range]: 17.0 [12.0–27.0]
vs. 33.0 [26.5–43.5], P = 0.019).
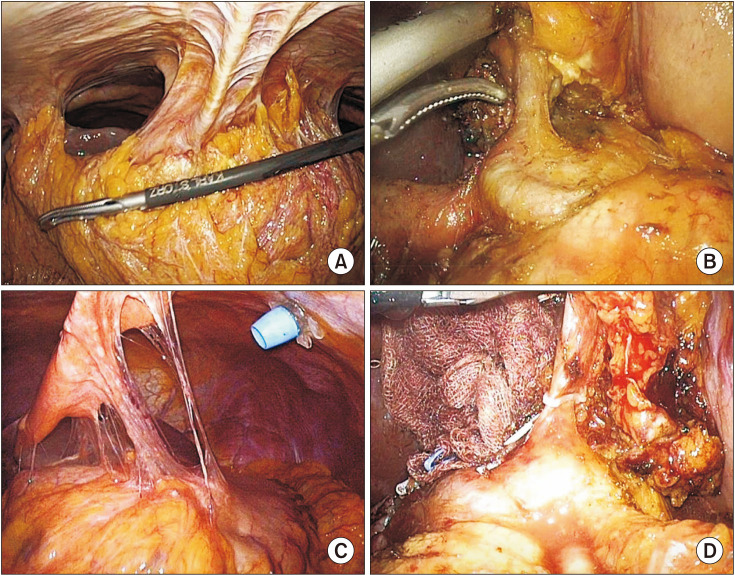 | Fig. 3Intraoperative findings of the second surgery. (A, C) Severe adhesion between omentum, liver, and gastric perforation site. (B, D) Second-tier lymph node dissection area without severe adhesion.
|
Median follow-up duration of 2-stage gastrectomy was 48 months. Only 1 patient had recurrence on remnant stomach after 41 months and survived 21 months after curative completion total gastrectomy before died for peritoneal seeding metastasis. Except for the patient, no patient was showed recurrence including the second-tier LN area. Survival analysis between the 2 groups was shown in Kaplan-Meier survival curve (
Fig. 4). Five-year overall survival (44.4%
vs. 100.0%, P = 0.113) and disease-free survival (52.2%
vs. 80.0%, P = 0.233) both were not significantly different between groups. However, there was less recurrence and death in the 2-stage gastrectomy group.
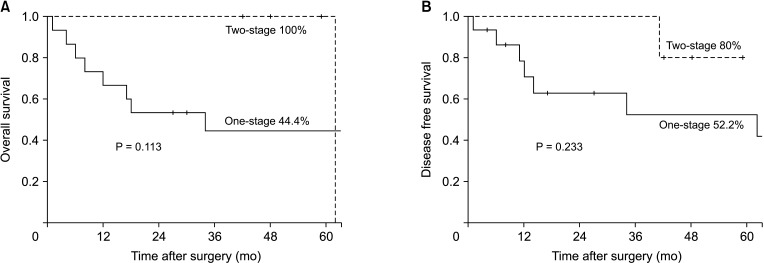 | Fig. 4Kaplan-Meier survival curve of perforated gastric cancer with 1-stage and 2-stage gastrectomy. (A) Five-year overall survival. (B) Five-year disease-free survival.
|
Go to :

DISCUSSION
Perforated gastric cancer is an unfamiliar surgical status developing in less than 5% of gastric cancers and is usually found in the advanced stage with severe complications compared to nonperforated status [
479131415161718]. The rate of early detection of gastric cancer has recently increased due to biennial endoscopy of the national cancer screening program and improvement of the health environment in Korea, and the numerical change in the rate of early detection of cancer over the past 10 years has reached 73.0% in 2010 from 28.6% in 1999 [
123]. Therefore, few cases of perforated gastric cancer were experienced and almost none of the research was studied so far in Korea, except 1 research which analyzed gastric cancer in both perforation and bleeding cases [
19]. In our institution, 22 patients (0.9%) were hospitalized in near 3 decades, which is an even lower rate than the average found in other studies, and we have successfully investigated the surgical outcomes of those patients [
7].
Two-stage gastrectomy was successfully performed in laparoscopy (6.7%
vs. 80.0%, P = 0.001) with favorable LN retrieval in our study results. Only 1 patient underwent open procedure for additional resection of paraaortic LN not because of technical problems. Two-stage gastrectomy was resulted in higher rate of D2 LND (60.0%
vs. 100.0%, P = 0.260) and higher number of retrieved LNs (median [range]: 17.0 [12.0–27.0]
vs. 33.0 [26.5–43.5], P = 0.019). There was no difference in overall survival and disease-free survival between the 2 groups, but the fine surgical quality may have influenced the higher survival rate of 2-stage gastrectomy. Several recent studies recommended 2-stage surgery for its advantage over conventional gastrectomy in oncological outcome with a higher rate of R0 resection and better survival rate, which was correlative to our study results [
4589]. The current study suggests that 2-stage surgery is a favorable surgical strategy in both acute peritonitis management and curative resection for the original disease with better longterm outcome.
Compared to other studies showing that perforation usually develops in the advanced stage, our study showed that 15.0% of patients had stage I and 33.0% had stage II, and 45.0% of patients had nonserosal invasion (pT1, pT2, and pT3) [
41618]. One previous study reported similar results of that 19% of patients had stage I and 12% had stage II [
13]. The gross pathologic type of 3 patients with pT2 was ulcerative infiltrating type (Bormann type 3), of which 1 was diagnosed with gastric ulcer within 6 months, and 1 patient with pT1 was excavated type (early gastric cancer type III). Despite the superficial layer invasion to mucosa or muscularis propria, the perforation might have been caused by an accompanying ulcer or ulcerative type of lesion, which is also indicated by the finding of gastric cancer in approximately 10% of gastric ulcer perforation [
15]. For this reason, perforated gastric cancer should not be regarded as far advanced stage cancer which needs palliative surgery, and more precise staging should be required to prepare an adequate surgical plan to manage on the oncological aspect. The time interval between 2-stage surgery gives the opportunity for proper evaluation and staging of cancer, whereas emergent 1-stage surgery has many limitations such as unavailability for the frozen section, intraoperative endoscopy, and undeciphered radiologic examination.
So far, only 1 study has analyzed the laparoscopy in perforated gastric cancer with 5 cases [
9], and its feasibility is yet assessed. Many surgeons still choose to rather perform laparotomy over laparoscopy because of technical failure in immediate management of peritonitis in perforated gastric cancer. Several benefits of laparoscopic management in perforated gastric cancer were identified at the edge of surgical outcomes in our study. First of all, exploration of the abdominal cavity was accomplished to determine the surgical stage of malignancy in first step surgery. Perforation of gastric cancer can occur at any stage, from an early stage where only D1 LND is required, to a highly advanced stage where inoperable factors exist. Diagnostic laparoscopy provides acquisition of the exact tumor stage by exploring the depth of serosal invasion, LN status, tumor appearance, and inoperable factors, such as small peritoneal seeding or small liver metastasis which radiological examination cannot evaluate. The surgical plan for curative resection of second step surgery is designed after then, whereas palliative management, such as chemotherapy, can be planned for the inoperable state of malignancy. The second point is that emergent management of acute peritonitis was also successfully conducted. A temporary gastrostomy with a Foley catheter, often used in severe gastric duodenal ulcer perforation, was able to address concerns about reperforation or another leakage that occurs after primary repair surgery [
2021]. As shown in
Fig. 1, a temporary gastrostomy with a Foley catheter is decided by the operator with consideration of the size, shape, and ulceration of the perforation. No complications occurred after the first step surgery, so we confirmed this laparoscopy of inflammation controlling surgery was able to convert perforation state into preperforation state without any concerns. Thirdly, the laparoscopic approach of first step surgery minimized tissue adhesion and increased the success rate of laparoscopic approach in the second step surgery. It is known that intra-abdominal adhesion starts to form after 7 days and continues until 4 weeks after abdominal surgery [
2223]. We attempted to shorten the interval time to less than 4 weeks when planning the second step of surgery, except in 2 patients who underwent the first surgery in other institutions. Consequently, we performed comparable rate of laparoscopic surgery in 2-stage surgery (6.7%
vs. 80.0%, P = 0.001) and did not show any difference from elective surgery in nonperforated gastric cancer on the aspect of surgical performance.
We recommend a few more steps to be followed in the future 2-stage gastrectomy. Preoperative or intraoperative endoscopy during the first surgery should be achieved for a more precise diagnosis. If preoperative endoscopy has the risk of worsening the patient's medical condition, postoperative endoscopy after first step surgery should be considered as shown in (
Fig. 2). Cytology examination should be also conducted in the first step of surgery to evaluate peritoneum dissemination not grossly visible. When locally advanced stage is found during abdominal exploration of the first surgery, the surgeon should consider neoadjuvant chemotherapy before the second step of curative gastrectomy [
2425].
Although not significant, 2-stage gastrectomy group showed a distinct advantage in both overall (44.4% vs. 100.0%, P = 0.113) and disease-free survival rate (52.2% vs. 80.0%, P = 0.233). The higher rate of D2 LND, which enabled curative resection, is considered to lead to a better prognosis of perforated gastric cancer, but other factors might have affected the prognosis as well. The surgical strategy of laparoscopy in 2-stage surgery improves the patient's outcome by less postoperative complications and faster recovery of general conditions after surgery when compared to open method. The higher rate of adjuvant chemotherapy in 2-stage gastrectomy group (66.7% vs. 80.0%, P > 0.999), although not in significant meaning, might be another reason for the better prognosis. Moreover, since there was time interval more than a decade between the 2 groups, it is also conceivable that the strategy of adjuvant chemotherapy in gastric cancer has advanced during this period.
This study had a few limitations. First of all, the study was a relatively long-term retrospective study due to low incidence and a small number of patients. Risk of differences in surgical technique, treatment strategy, and follow-up period is imposed on this factor and other unintentional selection biases also cannot be ruled out. Secondly, the number of cases is insufficient for the statistical analysis, especially for the statistical comparison between laparoscopic and nonlaparoscopic surgeries. Larger scale in multicenter research is further needed for more intensive analysis, such as Cox proportional hazard analysis for prognostic factors. In contrast, our study is a unique report focusing on the cases of perforated gastric cancer with the laparoscopic approach in 2-stage surgery.
In conclusion, the laparoscopic management in perforated gastric cancer provides beneficial gains in surgical performance. With the oncological advantages of 2-stage gastrectomy, the laparoscopic approach might be considered as one of the alternative treatments for perforated gastric cancer.
Go to :






 PDF
PDF Citation
Citation Print
Print








 XML Download
XML Download