1. Tepe G, Micari A, Keirse K, Zeller T, Scheinert D, Li P, et al. Drug-coated balloon treatment for femoropopliteal artery disease: the chronic total occlusion cohort in the IN.PACT Global Study. JACC Cardiovasc Interv. 2019; 12:484–493. PMID:
30846089.
2. Laird JR, Schneider PA, Tepe G, Brodmann M, Zeller T, Metzger C, et al. Durability of treatment effect using a drug-coated balloon for femoropopliteal lesions: 24-month results of IN.PACT SFA. J Am Coll Cardiol. 2015; 66:2329–2338. PMID:
26476467.
3. Laird JR, Yeo KK. The treatment of femoropopliteal in-stent restenosis: back to the future. J Am Coll Cardiol. 2012; 59:24–25. PMID:
22192664.
4. Torii S, Kolodgie FD, Virmani R, Finn AV. IN.PACT™ Admiral™ drug-coated balloons in peripheral artery disease: current perspectives. Med Devices (Auckl). 2019; 12:53–64. PMID:
30858737.
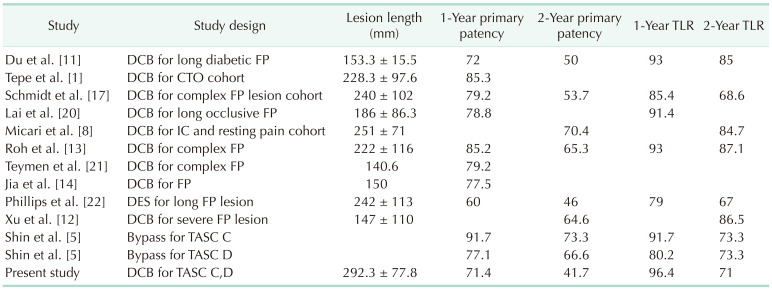
5. Shin SH, Kwon SH, Cho JH, Ahn HJ, Oh JH, Park HC. Outcomes of bypass surgery versus endovascular therapy for TASC II C and D femoro-popliteal lesions in patients with chronic limb ischemia. Korean J Vasc Endovasc Surg. 2010; 26:90–97.
6. Baril DT, Chaer RA, Rhee RY, Makaroun MS, Marone LK. Endovascular interventions for TASC II D femoropopliteal lesions. J Vasc Surg. 2010; 51:1406–1412. PMID:
20385464.

7. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007; 45 Suppl S:S5–S67. PMID:
17223489.

8. Micari A, Nerla R, Vadalà G, Castriota F, Grattoni C, Liso A, et al. 2-Year results of paclitaxel-coated balloons for long femoropopliteal artery disease: evidence from the SFA-long study. JACC Cardiovasc Interv. 2017; 10:728–734. PMID:
28385412.
9. Jaff MR, Rosenfield K, Scheinert D, Rocha-Singh K, Benenati J, Nehler M, et al. Drug-coated balloons to improve femoropopliteal artery patency: rationale and design of the LEVANT 2 trial. Am Heart J. 2015; 169:479–485. PMID:
25819854.

10. Zeller T, Rastan A, Macharzina R, Tepe G, Kaspar M, Chavarria J, et al. Drug-coated balloons vs. drug-eluting stents for treatment of long femoropopliteal lesions. J Endovasc Ther. 2014; 21:359–368. PMID:
24915582.

11. Du X, Wang F, Wu DM, Zhang MH, Jia X, Zhang JW, et al. Comparison between paclitaxel-coated balloon and standard uncoated balloon in the treatment of femoropopliteal long lesions in diabetics. Medicine (Baltimore). 2019; 98:e14840. PMID:
30921183.

12. Xu Y, Jia X, Zhang J, Zhuang B, Fu W, Wu D, et al. Drug-coated balloon angioplasty compared with uncoated balloons in the treatment of 200 Chinese patients with severe femoropopliteal lesions: 24-month results of AcoArt I. JACC Cardiovasc Interv. 2018; 11:2347–2353. PMID:
30448170.
13. Roh JW, Ko YG, Ahn CM, Hong SJ, Shin DH, Kim JS, et al. Risk factors for restenosis after drug-coated balloon angioplasty for complex femoropopliteal arterial occlusive disease. Ann Vasc Surg. 2019; 55:45–54. PMID:
30118857.

14. Jia X, Zhang J, Zhuang B, Fu W, Wu D, Wang F, et al. Acotec drug-coated balloon catheter: randomized, multicenter, controlled clinical study in femoropopliteal arteries: evidence from the AcoArt I trial. JACC Cardiovasc Interv. 2016; 9:1941–1949. PMID:
27659572.
15. Conte MS, Bradbury AW, Kolh P, White JV, Dick F, Fitridge R, et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg. 2019; 69(6S):3S–125S. PMID:
31159978.
16. Katsanos K, Spiliopoulos S, Kitrou P, Krokidis M, Karnabatidis D. Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2018; 7:e011245. PMID:
30561254.

17. Schmidt A, Piorkowski M, Görner H, Steiner S, Bausback Y, Scheinert S, et al. Drug-coated balloons for complex femoropopliteal lesions: 2-year results of a Real-World Registry. JACC Cardiovasc Interv. 2016; 9:715–724. PMID:
27056311.
18. TASC Steering Committee. Jaff MR, White CJ, Hiatt WR, Fowkes GR, Dormandy J, et al. An update on methods for revascularization and expansion of the TASC lesion classification to include below-the-knee arteries: a supplement to the inter-society consensus for the management of peripheral arterial disease (TASC II). Vasc Med. 2015; 20:465–478. PMID:
26268268.

19. Micari A, Brodmann M, Keirse K, Peeters P, Tepe G, Frost M, et al. Drug-coated balloon treatment of femoropopliteal lesions for patients with intermittent claudication and ischemic rest pain: 2-year results from the IN.PACT Global Study. JACC Cardiovasc Interv. 2018; 11:945–953. PMID:
29798770.
20. Lai Z, Zhang X, Shao J, Li K, Fang L, Xu L, et al. One-year results of drug-coated balloons for long and occlusive femoropopliteal artery disease: a single-arm trial. BMC Cardiovasc Disord. 2020; 20:65. PMID:
32028896.

21. Teymen B, Akturk S, Akturk U, Tdjani M. Comparison of drug-eluting balloon angioplasty with sel f-expanding interwoven nitinol stent deployment in patients with complex femoropopliteal lesions. Vascular. 2018; 26:54–61. PMID:
28708023.
22. Phillips JA, Falls A, Kolluri R, Whipp A, Collins C, Mohir-Sadaai S, et al. Full drug-eluting stent jacket: two-year results of a single-center experience with Zilver PTX stenting for long lesions in the femoropopliteal arteries. J Endovasc Ther. 2018; 25:295–301. PMID:
29544372.

23. Schillinger M, Sabeti S, Loewe C, Dick P, Amighi J, Mlekusch W, et al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med. 2006; 354:1879–1888. PMID:
16672699.

24. Schneider PA. Evolution and current use of technology for superficial femoral and popliteal artery interventions for claudication. J Vasc Surg. 2017; 66:916–923. PMID:
28842077.

25. Ueda M, Becker AE, Tsukada T, Numano F, Fujimoto T. Fibrocellular tissue response after percutaneous transluminal coronary angioplasty: an immunocytochemical analysis of the cellular composition. Circulation. 1991; 83:1327–1332. PMID:
2013150.

26. Kim MS, Joo YS, Park KH. Results of simultaneous hybrid operation in multi-level arterial occlusive disease. J Korean Surg Soc. 2010; 79:386–392.

27. Kim D, Bresette C, Liu Z, Ku DN. Occlusive thrombosis in arteries. APL Bioeng. 2019; 3:041502. PMID:
31768485.

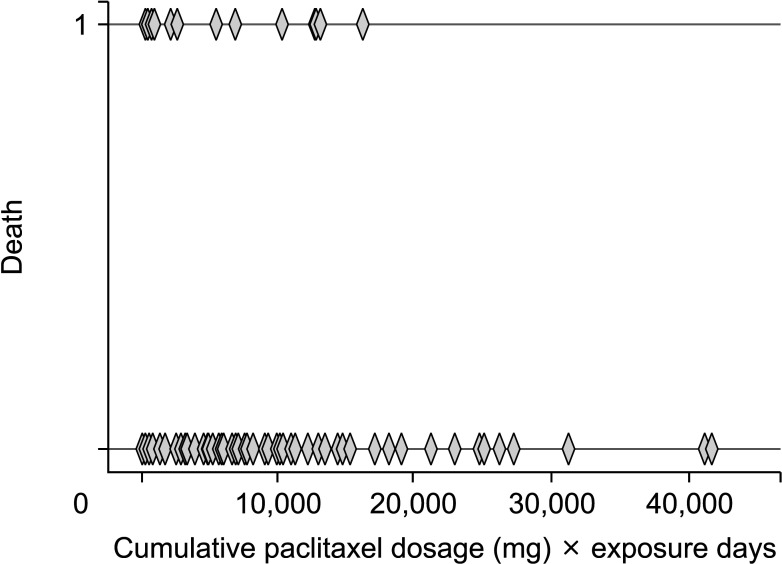
28. Swaminathan RV, Jones WS, Patel MR. Is paclitaxel causing mortality during lower-extremity revascularization? J Am Heart Assoc. 2019; 8:e012523. PMID:
31094266.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download