Abstract
Purpose
Treatment with 4 cycles of docetaxel and cyclophosphamide (TC) in the adjuvant setting is associated with better outcomes than treatment with doxorubicin and cyclophosphamide (AC). However, Western guidelines have indicated that TC confers a high risk (>20%) of febrile neutropenia (FN), while AC confers an intermediate risk (10%–20%) of FN. Threrefore, we evaluated the incidence of FN and the clinical utilization of pegfilgrastim prophylaxis after adjuvant TC chemotherapy.
Methods
We categorized 201 patients who received adjuvant TC chemotherapy into 3 groups according to the method of prophylaxis and compared neutropenic events, other adverse events, and hospital care costs in the 3 groups.
Results
The incidence of grade 4 neutropenia decreased from 93.0% in patients without prophylaxis to 82.4% in those who received secondary prophylaxis and 16.7% in those who received primary prophylaxis. Although the incidence of FN was not different between patients without prophylaxis and patients who received secondary prophylaxis (15.7% and 14.9%), none of the patients who received primary prophylaxis developed FN. Moreover, a decrease in neutropenic events resulted in a significant decrease in the mean duration of neutropenia (2.50 days to 0.08 days, P < 0.001), the risk of hospitalization (29.8% to 2.2%, P < 0.001), and the mean total hospital care cost for all chemotherapy cycles (790.80 to 486.00 US dollars, P < 0.001).
Adjuvant chemotherapy for early-stage breast cancer (EBC) reduces recurrence rates and improves survival rates [1]. Among various adjuvant chemotherapy regimens, anthracycline-based chemotherapy remains the core of most adjuvant chemotherapy regimens for EBC. However, because cardiotoxicity and secondary leukemia have been associated with anthracycline-based regimens, the development of the adjuvant chemotherapy landscape for EBC is ongoing [234]. Since 2005, when studies suggested that taxane-based regimens without anthracycline might provide equivalent or superior results to anthracycline-based regimens [5], the use of anthracycline-based regimens has declined while the use of taxane-based regimens has increased among patients with breast cancer in the United States [6].
Previous studies indicate that taxane-based regimens have become the standard for adjuvant chemotherapy. Treatment with 4 cycles of docetaxel and cyclophosphamide (TC) in the adjuvant setting was shown to be associated with significant improvements in 5-year disease-free survival (DFS) and overall survival (OS) over doxorubicin and cyclophosphamide (AC) in a phase III randomized clinical trial [7]. After that, second-generation phase III trials demonstrated that survival outcomes for 6 cycles of TC and 4 cycles of epirubicin + cyclophosphamide followed by 4 cycles of docetaxel were equally excellent in human epidermal growth factor receptor (HER) 2-negative EBC with lower risk [89]. Thus, the TC regimen is attracting attention for adjuvant chemotherapy of EBC.
Febrile neutropenia (FN) is a serious adverse effect encountered in patients undergoing myelosuppressive chemotherapy for EBC [10]. Because chemotherapy-induced FN is associated with life-threatening infections, prolonged hospitalization, increased health care costs, and modification of the dose or schedule of chemotherapy, it is critical to assess the risk of FN and prevent it with recombinant granulocyte colony-stimulating factor (G-CSF) in a myelosuppressive chemotherapy regimen [11]. The National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology for Hematopoietic Growth Factors indicate that TC confers a high risk (>20%) of FN and recommends the use of primary prophylactic G-CSF [12]. In a meta-analysis with 902 patients from 13 studies, the estimated rate of FN without primary G-CSF was 29.1%, while that with primary G-CSF prophylaxis was 6.8% for TC [13]. However, in an early phase III randomized clinical trial, treatment with 4 cycles of adjuvant TC was associated with a 5% risk of FN [5]. Moreover, in a randomized study conducted in Japan, treatment with 6 cycles of neoadjuvant TC was associated with a 13.8% risk of FN [14].
Considering the ethnic differences in hematologic toxicity and the absence of any Korean reports on FN risk after adjuvant TC chemotherapy, we evaluated the incidence of chemotherapy-related neutropenic events and other adverse events during adjuvant TC chemotherapy in Korean patients with EBC. Furthermore, we assessed the clinical utilization of primary or secondary prophylactic support with long-acting G-CSF (pegfilgrastim) in these patients.
This study was approved by the Institutional Review Board of The Catholic University of Korea, St. Vincent's Hospital (No. VC18RESI0162). Written informed consent was obtained from all patients. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The electronic medical records were reviewed for patients with EBC who received adjuvant TC chemotherapy from July 2015 to December 2019.
In total, 209 consecutive patients who received adjuvant TC chemotherapy were included in this study. Eight patients, including 2 patients who did not complete adjuvant TC chemotherapy and 6 patients treated with neoadjuvant chemotherapy, were excluded to minimize other confounding factors. A total of 201 patients were included in the current study.
We reviewed the patients' demographics and tumor characteristics, including age, body mass index (BMI [kg/m2]), body surface area (BSA [m2]), menopausal status, type of surgery, pathological T and N staging, histologic grade and type, hormone receptor (HR) and HER2 expression, and comorbidities. HR status was determined using an enzyme immunoassay and reported in the patients' medical records. Immunohistochemistry (IHC), fluorescence in situ hybridization (FISH), or silver in situ hybridization (SISH) was used to evaluate HER2 status, and an IHC score of 0 or +1 or an IHC score of +2 and negative FISH/SISH were defined as negative HER2 overexpression.
All patients received a total of 4 cycles of TC chemotherapy, 3 weeks apart. In each cycle, docetaxel (75 mg/m2, intravenous [IV] for 1 hour) was initially administered, immediately followed by cyclophosphamide (600 mg/m2, IV for 1 hour). Secondary prophylaxis using pegfilgrastim (Neulasta, Amgen, Thousand Oaks, CA, USA) has been used in Korea since 2015, when it became covered by the National Health Insurance. Secondary prophylaxis with pegfilgrastim was defined as the use of G-CSF if a patient experienced a neutropenic event in the previous chemotherapy cycle. Primary prophylaxis using pegfilgrastim has been used in Korea since April 2018 with the approval of the National Health Insurance program. Pegfilgrastim was administered subcutaneously between 24 and 48 hours after the administration of chemotherapy. When not using pegfilgrastim, short-acting recombinant G-CSF (filgrastim) was administered daily after each cycle for grade 3 or 4 neutropenia until the absolute neutrophil count (ANC) was restored to 1,000/mm3. Laboratory tests, including complete blood cell (CBC) counts with differential and chemistry assays, were checked before each chemotherapy cycle and on day 6. After chemotherapy, baseline CBC counts were measured from day 6 until the ANC was restored to 1,000/mm3. All patients with FN received prophylactic antibiotic therapy comprising 1-g IV cefoperazone twice daily and 200-mg tobramycin sulfate once daily, unless contraindicated.
The incidence of FN and FN-related complications according to the Common Terminology Criteria for Adverse Events (CTCAE, version 4.02) were investigated. FN was defined as neutropenia (grade 4 or grade 3 for over 48 hours) with a febrile event (oral temperature of ≥38.3℃, or ≥38.0℃ for over 1 hour) observed by medical staff. Dose reduction was defined as a reduction in the delivered dosage(s) of agent(s) administered relative to the standard values, and dose delay was defined as a chemotherapy interval of more than 21 days. The chemotherapy relative dose intensity (RDI) was estimated based on the ratio of delivered dose intensity and the reference standard dose intensity [15]. Total hospital care cost was calculated as the costs associated with all medical claims during the entire cycle or within each cycle. Outpatient hospital visit costs, hospitalization costs, chemotherapy costs, and G-CSF costs were all included in the total hospital care cost measure. The costs represented the reimbursed amount paid by the patient, as documented in the electronic medical record.
The chi-square test and Fisher exact test were used to determine differences in categorical variables between groups. The unpaired t-test and analysis of variance were used for comparison between continuous and independent variables that follow a normal distribution (age, BMI, BSA, RDI, recovery from neutropenia [days]). Continuous and independent variables that do not follow a normal distribution were analyzed using Mann-Whitney tests (weight gain [kg]). A P-value of <0.05 was considered to be statistically significant. The analyses were performed using PASW Statistics, ver. 18.0 for Windows (IBM Corp., Armonk, NY, USA).
Between July 2015 and December 2019, 201 Korean patients (804 cycles) with EBC who received adjuvant TC chemotherapy were included in the analysis. A total of 115 patients (57.2%) did not receive prophylaxis with pegfilgrastim, 74 (36.8%) received secondary prophylaxis, and 12 (6.0%) received primary prophylaxis with pegfilgrastim during adjuvant TC chemotherapy. The demographics and clinical characteristics of the study population by the method of prophylaxis with pegfilgrastim are shown in Table 1. Overall, the median age was 55 years (range, 21–79 years). A total of 37 patients (18.4%) were older than 65 years. The mean BMI and BSA were 24.71 ± 3.48 kg/m2 and 1.60 ± 0.13 m2, respectively. Patients who received primary prophylaxis had a significantly older median age at diagnosis than patients who did not receive prophylaxis or received secondary prophylaxis (P = 0.009). There were no significant differences in menopausal status, type of surgery, tumor stage, histologic grade, histologic type, comorbidity, or HR or HER2 status among the 3 groups (Table 1).
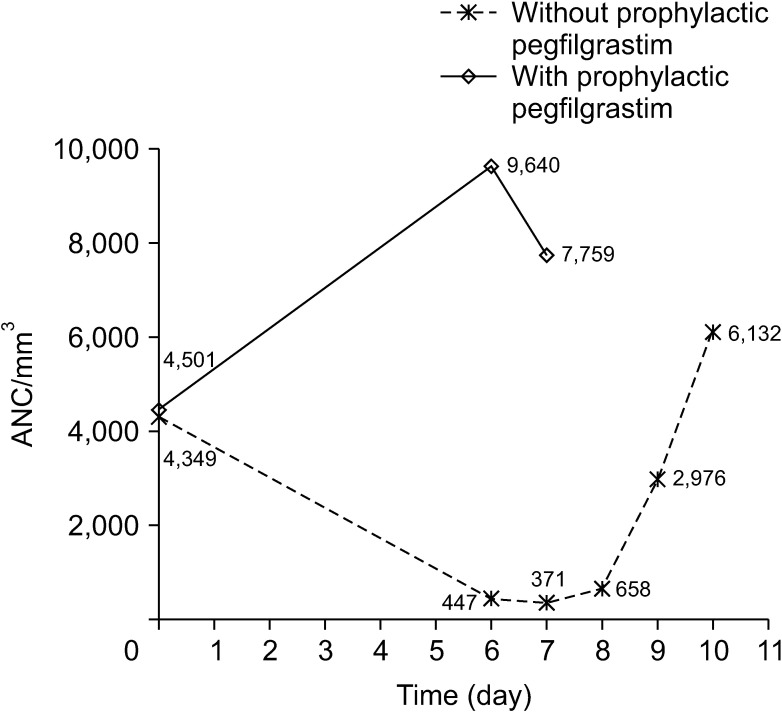
The combined incidence of grade 3 and 4 neutropenia was 3.5%, and 93.0% of patients did not receive prophylaxis with pegfilgrastim (Table 2). However, the incidence of grade 4 neutropenia decreased to 82.4% with secondary prophylaxis (P = 0.035) and 16.7% with primary prophylaxis (P < 0.001). In the analysis of 804 chemotherapy cycles, the incidence of grade 4 neutropenia was 74.6% in all cycles without prophylaxis and 5.3% in all cycles with prophylaxis with pegfilgrastim (P < 0.001) (Table 3). Moreover, the mean period of recovery from neutropenia was significantly shorter in chemotherapy cycles with prophylaxis with pegfilgrastim than in chemotherapy cycles without prophylaxis (2.50 ± 1.09 days vs . 0.08 ± 0.26 days, P < 0.001) (Table 3). The ANC changes after the chemotherapy cycle according to pegfilgrastim prophylaxis status are shown in Fig. 1.
The incidence of FN was not significantly different between patients who did not receive prophylaxis and patients who received secondary prophylaxis (15.7% and 14.9%, P = 0.528) (Table 2). However, no patients who received primary prophylaxis with pegfilgrastim developed FN. Overall, FN occurred in 6.9% of all chemotherapy cycles without prophylaxis and 0.9% of all cycles with prophylaxis with pegfilgrastim (P < 0.001) (Table 3).
Regarding hematologic toxicities other than neutropenia, there were no differences in the incidence of anemia, thrombocytopenia, and transfusion between patients who did not receive prophylaxis and patients with secondary prophylaxis (Table 2). These findings were the same when the results were analyzed by chemotherapy cycle (Table 3). There was no anemia or thrombocytopenia in patients who received primary prophylaxis. Moreover, the weight gained due to chemotherapy was less in patients who received primary prophylaxis than in the other 2 groups (Table 2). Among all patients who received TC chemotherapy, no patient experienced severe hepatotoxicity or nephrotoxicity.
Among the 115 patients who did not receive prophylaxis with pegfilgrastim, 6 (5.2%) developed neutropenic infections, which included 2 patients with chemoport infection and 4 patients with wound infections. Among the 74 patients who received secondary prophylaxis, 5 (6.8%) developed neutropenic infections, which included 1 patient with a chemoport infection and 4 patients with wound infections. Among patients who received primary prophylaxis, there were no neutropenia-associated infections (Table 2).
Although there were no significant differences in dose reduction (2.6% vs. 2.7%, P = 0.651) or treatment delay (3.5% vs. 1.4%, P = 0.078) between patients who did not receive prophylaxis and patients who received secondary prophylaxis, the RDI was lower in patients who did not receive prophylaxis than in those who received secondary prophylaxis (99.33% vs. 99.69%, respectively; P = 0.025). In patients who received primary prophylaxis, there was no association between treatment and dose reduction and treatment delay, and the RDI was 100% (Table 2).
Compared with treatment without prophylaxis, patients who received secondary prophylaxis were not associated with a reduction in the risk of hospitalization (37.4% vs. 31.1%, P = 0.233), whereas primary prophylaxis was significantly associated with a reduction in the risk of hospitalization compared with the other 2 groups (37.4% vs . 8.3%, P = 0.038; 31.1% vs. 8.3%, P = 0.045) (Table 2). The incidence of hospitalization in each chemotherapy cycle was 29.8% in patients who did not receive prophylaxis and 2.2% in patients who received pegfilgrastim prophylaxis (P < 0.001) (Table 3).
The mean total hospital care cost for all chemotherapy cycles was greater for patients who did not receive prophylaxis than for patients who received secondary prophylaxis (790.80 US dollars [USD] vs. 728.40 USD, P = 0.008). In patients who received primary prophylaxis, the mean total hospital care cost for all chemotherapy cycles was 486.00 USD, and this cost was significantly lower than that for the other 2 groups (P < 0.001). In the analysis of each chemotherapy cycle, the mean hospital care cost for each chemotherapy cycle was significantly greater for patients who did not receive prophylaxis than for patients who received prophylactic pegfilgrastim (199.20 USD vs. 157.20 USD, P < 0.001).
In this study, we assessed the clinical effectiveness of pegfilgrastim prophylaxis in adjuvant TC chemotherapy by directly comparing the incidences of chemotherapy-related neutropenic events and other adverse events according to the method of prophylaxis in Korean EBC patients who received adjuvant TC chemotherapy. Primary prophylaxis with pegfilgrastim after adjuvant TC chemotherapy was significantly associated with a decrease in the incidence of chemotherapy-related neutropenic events, including FN, and the mean period of recovery from neutropenia, the risk of hospitalization, and the cost of hospital care for chemotherapy compared to those in patients who did not receive prophylaxis or who received secondary prophylaxis.
With longer follow-up, 4 cycles of adjuvant TC chemotherapy showed a significant benefit over 4 cycles of AC chemotherapy in regard to DFS and OS and had a lower risk of anthracycline-related cardiac toxicity than the AC regimen [7]. Although the TC regimen has these clinical benefits over the AC regimen, the TC regimen results in a significantly higher incidence of chemotherapy-induced hematologic toxicities, such as neutropenia and FN, than the AC regimen [71316]. In a previous meta-analysis with 902 patients from 13 studies and with 2,532 patients from 14 studies, the estimated FN rates without primary G-CSF were 29.1% and 31.3% [1316]. Furthermore, the NCCN guidelines have indicated that TC confers a high risk (>20% chance of occurrence) of FN, while AC confers an intermediate risk (10%–20% chance) of FN [12]. However, the incidence of FN after adjuvant TC chemotherapy was not reported in more than 20% of all studies. The clinical trial conducted by the US Oncology Group reported a 5% FN incidence [5], and the West German Study PlanB trial reported a 6% FN incidence without primary prophylaxis [9].
In this study, the overall incidence rates of grade 4 neutropenia and FN were 93.0% and 15.7%, respectively, in patients who did not receive prophylaxis with pegfilgrastim after adjuvant TC chemotherapy. The incidence of grade 4 neutropenia in the present study was a significantly higher than that observed in previous studies conducted in Western countries (10.7%–50.8%) [5917]. However, the incidence of FN in the present study was rather low compared to that observed in 2 previous meta-analyses [1316]. Although it is difficult to explain the exact reason why the incidence rates of FN were low compared to those of the previous 2 meta-analyses and previous studies conducted in Western countries, ethnic differences in hematologic toxicity from docetaxel-based chemotherapy may be an important factor. The pharmacokinetics of docetaxel exhibit wide interindividual variability, which might lead to poor predictability of treatment-related side effects and outcomes [181920]. This variability of docetaxel pharmacokinetics or pharmacodynamics was also seen in a study conducted exclusively in Asian patients, including 103 Chinese, 111 Malay, and 73 Indian patients [19]. In a randomized study conducted in Japan, treatment with 6 cycles of neoadjuvant TC was associated with a 13.8% risk of FN [14]. Moreover, the incidence of FN was 25.2% for 4 cycles of the AC regimen and 4.7% for 4 cycles of the docetaxel regimen (75 or 100 mg/m2) in a Korean study on sequential AC and docetaxel chemotherapy [21].
The use of long-acting G-CSF results in better supportive care and improved quality of life in breast cancer patients by significantly reducing grade 4 neutropenia and FN [10111316]. In our study, the incidence of grade 4 neutropenia decreased from 93.0% to 16.7%, and the incidence of FN decreased from 15.7% to 0%, among all patients who received primary prophylactic pegfilgrastim. Moreover, a decrease in the incidence of grade 4 neutropenia and FN resulted in a significant decrease in the mean duration of neutropenia (from 2.50 days to 0.08 days), the risk of hospitalization (from 29.8% to 2.2%), and the mean total hospital care cost for all chemotherapy cycles (from 790.80 to 486.00 USD). The rate of hospitalization after the use of primary prophylactic pegfilgrastim in our current study was much lower than the rate of hospitalization adjusted for G-CSF primary prophylaxis observed in a previous study (6.7%–13.1%) [22]. Furthermore, the use of long-acting G-CSF results in the preservation of RDI, which is an important factor in achieving optimal survival outcomes after adjuvant chemotherapy. In this study, the RDI was significantly higher in patients who received primary prophylaxis than in patients who did not receive prophylactic pegfilgrastim (99.33% vs . 100%, P = 0.049). Although our current study did not analyze survival outcomes due to the short follow-up period, further studies with long-term follow-up will provide conclusions about improving survival outcomes with the use of long-acting G-CSF.
Our study has some limitations, such as its retrospective nature. The number of patients was small because only patients who received adjuvant TC chemotherapy at a single institution were included. Moreover, decisions regarding hospitalization, dose reduction, and treatment delays were made based on our institutional treatment protocol. Additionally, only FN observed by medical staff was counted in the current study. Therefore, the incidence of FN may have been underestimated, as febrile events confirmed by the patient prior to the hospital visit were not included. However, we believe that this study has clinical value because it is the first study assessing the incidence of chemotherapy-related neutropenic events and other adverse events, and the clinical utilization of primary or secondary prophylactic support with long-acting G-CSF (pegfilgrastim) during adjuvant TC chemotherapy, in Korean patients with EBC.
In summary, our study demonstrated that the overall incidence of grade 4 neutropenia, at 93.0%, was significantly higher than that observed in previous studies conducted in Western countries. Although the incidence of FN was 15.7%, rather low compared to that observed in the previous 2 meta-analyses, the use of pegfilgrastim prophylaxis during adjuvant TC chemotherapy was associated with significant decreases in the incidence of grade 4 neutropenia, FN, the risk of hospitalization, and the cost of hospital care for chemotherapy compared to no prophylaxis. Further large-scale prospective studies will help fill the gap in the evidence regarding FN risk and will thus inform the use of pegfilgrastim prophylaxis for this regimen in real-world practices.
References
1. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005; 365:1687–1717. PMID: 15894097.
2. Smith LA, Cornelius VR, Plummer CJ, Levitt G, Verrill M, Canney P, et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer. 2010; 10:337. PMID: 20587042.

3. Pal SK, Childs BH, Pegram M. Emergence of nonanthracycline regimens in the adjuvant treatment of breast cancer. Breast Cancer Res Treat. 2010; 119:25–32. PMID: 19795206.

4. Smith RE, Bryant J, DeCillis A, Anderson S. National Surgical Adjuvant Breast and Bowel Project Experience. Acute myeloid leukemia and myelodysplastic syndrome after doxorubicin-cyclophosphamide adjuvant therapy for operable breast cancer: the National Surgical Adjuvant Breast and Bowel Project experience. J Clin Oncol. 2003; 21:1195–1204. PMID: 12663705.

5. Jones SE, Savin MA, Holmes FA, O'Shaughnessy JA, Blum JL, Vukelja S, et al. Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol. 2006; 24:5381–5387. PMID: 17135639.

6. Giordano SH, Lin YL, Kuo YF, Hortobagyi GN, Goodwin JS. Decline in the use of anthracyclines for breast cancer. J Clin Oncol. 2012; 30:2232–2239. PMID: 22614988.

7. Jones S, Holmes FA, O'Shaughnessy J, Blum JL, Vukelja SJ, McIntyre KJ, et al. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of us oncology research trial 9735. J Clin Oncol. 2009; 27:1177–1183. PMID: 19204201.

8. Blum JL, Flynn PJ, Yothers G, Asmar L, Geyer CE Jr, Jacobs SA, et al. Anthracyclines in early breast cancer: the ABC Trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG Oncology). J Clin Oncol. 2017; 35:2647–2655. PMID: 28398846.

9. Nitz U, Gluz O, Clemens M, Malter W, Reimer T, Nuding B, et al. West German Study PlanB trial: adjuvant four cycles of epirubicin and cyclophosphamide plus docetaxel versus six cycles of docetaxel and cyclophosphamide in HER2-negative early breast cancer. J Clin Oncol. 2019; 37:799–808. PMID: 30785826.

10. Dale DC. Colony-stimulating factors for the management of neutropenia in cancer patients. Drugs. 2002; 62 Suppl 1:1–15.

11. Kuderer NM, Dale DC, Crawford J, Lyman GH. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systemat ic review. J Cl in Oncol. 2007; 25:3158–3167.
12. National Comprehensive Cancer Networt (NCCN). Clinical practice guidelines in oncology for hematopoietic growth factors (NCCN Guidelines version 2.2020) [Internet]. Fort Washington, PA: NCCN;2020. cited 2020 Apr 7. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
13. Younis T, Rayson D, Thompson K. Primary G-CSF prophylaxis for adjuvant TC or FEC-D chemotherapy outside of clinical trial settings: a systematic review and meta-analysis. Support Care Cancer. 2012; 20:2523–2530. PMID: 22252548.

14. Ishiguro H, Masuda N, Sato N, Higaki K, Morimoto T, Yanagita Y, et al. A randomized study comparing docetaxel/cyclophosphamide (TC), 5-fluorouracil/epirubicin/cyclophosphamide (FEC) followed by TC, and TC followed by FEC for patients with hormone receptor-positive HER2-negative primary breast cancer. Breast Cancer Res Treat. 2020; 180:715–724. PMID: 32170634.

15. Weycker D, Barron R, Edelsberg J, Kartashov A, Lyman GH. Incidence of reduced chemotherapy relative dose intensity among women with early stage breast cancer in US clinical practice. Breast Cancer Res Treat. 2012; 133:301–310. PMID: 22270932.

16. Fernandes R, Mazzarello S, Stober C, Vandermeer L, Dudani S, Ibrahim MF, et al. Optimal primary febrile neutropenia prophylaxis for patients receiving docetaxel-cyclophosphamide chemotherapy for breast cancer: a systematic review. Breast Cancer Res Treat. 2017; 161:1–10. PMID: 27783280.

17. Li Y, Family L, Yang SJ, Klippel Z, Page JH, Chao C. Risk of febrile neutropenia associated with select myelosuppressive chemotherapy regimens in a large community-based oncology practice. J Natl Compr Canc Netw. 2017; 15:1122–1130. PMID: 28874597.

18. Baker SD, Li J, ten Tije AJ, Figg WD, Graveland W, Verweij J, et al. Relationship of systemic exposure to unbound docetaxel and neut ropenia. Cl in Pharmacol Ther. 2005; 77:43–53.
19. Hor SY, Lee SC, Wong CI, Lim YW, Lim RC, Wang LZ, et al. PXR, CAR and HNF4alpha genotypes and their association with pharmacokinetics and pharmacodynamics of docetaxel and doxorubicin in Asian patients. Pharmacogenomics J. 2008; 8:139–146. PMID: 17876342.
20. Onoue H, Yano I, Tanaka A, Itohara K, Hanai A, Ishiguro H, et al. Significant effect of age on docetaxel pharmacokinetics in Japanese female breast cancer patients by using the population modeling approach. Eur J Clin Pharmacol. 2016; 72:703–710. PMID: 26905999.

21. Kim CG, Sohn J, Chon H, Kim JH, Heo SJ, Cho H, et al. Incidence of febrile neutropenia in Korean female breast cancer patients receiving preoperative or postoperative doxorubicin/cyclophosphamide followed by docetaxel chemotherapy. J Breast Cancer. 2016; 19:76–82. PMID: 27064666.

22. Barcenas CH, Niu J, Zhang N, Zhang Y, Buchholz TA, Elting LS, et al. Risk of hospitalization according to chemotherapy regimen in early-stage breast cancer. J Clin Oncol. 2014; 32:2010–2017. PMID: 24868022.

Fig. 1
The absolute neutrophil count (ANC) changes after chemotherapy according to the use of prophylaxis with pegfilgrastim.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download