Abstract
Purpose
Single-incision laparoscopic distal gastrectomy (SIDG) requires experienced camera operators for a stable image. Since it is difficult for skilled camera operators to participate in all SIDG, we began performing solo surgery using mechanical camera holders. We aimed to compare the short-term outcomes and cost between solo SIDG and conventional multiport laparoscopic distal gastrectomy (MLDG) for early gastric cancer (EGC).
Methods
From January 2014 to December 2016, a total of 938 consecutive patients underwent laparoscopic gastrectomy for EGC. Solo SIDG (n = 99) and MLDG patients (n = 198) were selected and 1:2 propensity score matching was done to compare the quality of operation and cost-effectiveness. All solo SIDG was performed by a surgeon using a camera holder, without any assistant.
Results
Mean operation time (120 ± 35.3 vs. 178 ± 53.4 minutes, P = 0.001) and estimated blood loss (24.6 ± 47.4 vs. 46.7 ± 66.5 mL, P = 0.001) were significantly lower in the solo SIDG group. Hospital stay, use of analgesics, and postoperative inflammatory markers (WBC, CRP) were similar between the 2 groups. The early (<30 days) complication rate in solo SIDG and MLDG groups was 21.2% and 23.7%, respectively (P = 0.240); the late (≥30 days) complication rate was 7.1% and 11.1%, respectively (P = 0.672). The manpower cost of solo SIDG was significantly lower than that of MLDG (P = 0.001).
Early gastric cancer (EGC) accounts for more than 60% of gastric cancer incidence in Korea. With the development of laparoscopic skills and equipment, current efforts in laparoscopic gastrectomy have shifted toward reducing wound size and postoperative pain. Since the first report on single-incision laparoscopic distal gastrectomy (SIDG) by Omori et al. [1] in 2012, SIDG was utilized as a treatment option for EGC. Pure SIDG without any additional ports was performed by Ahn et al. [2] in 2014. They reported that pure SIDG is both safe and feasible for EGC and has similar operation time and better short-term outcomes than conventional multiport laparoscopic distal gastrectomy (MLDG) in terms of postoperative pain, estimated blood loss (EBL), inflammatory reaction, and cosmetic result. Omori et al. [3] also concluded that SIDG with D2 lymphadenectomy vs. MLDG showed similar 5-year overall survival (93.7% vs. 87.6%; P = 0.689) and recurrence-free survival rates (90.0% vs. 87.6%; P = 0.958).
Despite the positive reports, unlike single-incision laparoscopic appendectomy, cholecystectomy, or colectomy, SIDG is still applied only in limited centers mainly due to its technical difficulties and lack of data on long-term oncologic outcomes [4,5,6,7]. When performing SIDG, the patient needs to be placed in a lithotomy position with both the operator and the camera operator sitting together between the patient's legs, which limits movement. This exacerbates the clashing of instruments, which is a major limitation of SIDG. Hence, an experienced camera operator is needed to maintain a stable surgical field. To overcome the spatial constraints and resolve the limited availability of skilled camera operators, we began solo surgery with a mechanical camera holder in 2014. Solo surgery is defined as a surgery where the surgeon alone manipulates all instruments including the camera to avoid communication problems and unnecessary camera movements [8]. Since the first introduction of solo surgery in 2000 [9], its application has increased into many fields of abdominal surgeries.
There is, however, little information concerning outcomes of solo SIDG in the literature. Therefore, we aimed to confirm safety and feasibility of solo SIDG by investigating the short-term operative outcomes and cost between solo SIDG and MLDG for EGC.
A total of 1,322 consecutive patients with gastric cancer underwent laparoscopic distal gastrectomy at our institution from January 2014 to December 2016. Prospectively recorded data of these patients were reviewed. All operations were performed by the same surgical team.
We extracted the data of 124 and 1,198 patients who underwent solo SIDG and MLDG, respectively. The inclusion criteria for this study were as follows; patients with clinically diagnosed EGC and pathologically proven stage I–II gastric cancer, having no other malignancy, have received more than D1 (lymph node number 1, 3, 4sb, 4d, 5, 6, 7 according to Japanese Gastric Cancer treatment guidelines in 2014) lymph node dissection, and have received R0 surgery. Patients who received neoadjuvant chemotherapy or adjuvant chemotherapy were excluded. Finally, 938 patients (solo SIDG, 103; MLDG, 835) were analyzed for the study.
To reduce bias from confounding variables, we performed propensity score matching (PSM) [10]. Propensity scores were obtained using binary logistic regression with covariates of age, sex, body mass index (BMI), American Society of Anesthesiologists physical status (ASA PS) classification [11], abdomen operation history, location of the main lesion, maximum tumor size, and clinical stage. Patients who underwent solo SIDG were matched to patients who underwent MLDG at 1:2 ratio using the PSM method (solo SIDG, 99; MLDG, 198).
There was no distinctive indication for performing solo SIDG, although the initial preference of patient characteristics was cases of EGC with low BMI. After gaining experience, solo SIDG was performed regardless of patient BMI.
The protocol of this retrospective cohort study was approved by the Institutional Review Board of Seoul National University Bundang Hospital, Korea, an academic hospital affiliated with Seoul National University College of Medicine (No. B-1711-430-103). Written informed consent was waived due to its retrospective nature.
Detailed surgical procedure was explained in our previous study [12]. To briefly summarize the procedures, the patient was placed in a reverse Trendelenburg lithotomy position. A manually controlled passive scope holder was used, with its base fixed on the left side of the patient at the pelvis level. A transumbilical 2.5 cm incision was made on the skin, with a larger incision of about 4 cm in the fascia. During dissection, scope manipulation is done using the right hand while the left hand, usually retracting tissue using a grasper, stays still in the field. Distal gastrectomy needs only about 10 major fields of view, and movement of the scope within the major viewpoints can be done finely by adjusting the knobs of the flexible scope.
The following variables were analyzed to evaluate the quality of the operations; operation time, EBL, transfusion, conversions to an open procedure, number of retrieved lymph nodes, type of reconstruction method, morbidity, and mortality. Postoperative complications were classified as “early (<30 days)” and “late (≥30 days)” according to the time of onset. Morbidity was graded based on the Clavien-Dindo classification [13].
The 2 groups received the same clinical pathway for postoperative management. If there were no complications, patients were permitted sips of water (SOW) 1 day after surgery, and with no trouble after SOW, a solid fluid diet (SFD) was given the next day. Laboratory examinations were performed on postoperative days 2 and 4 and were compared with preoperative values if necessary. If the patient had no complaint after soft diet and showed no postoperative complications, they were discharged on postoperative day 5 or 6.
All patients were equipped with a fentanyl intravenous (IV) patient-controlled analgesia (PCA) system for 48–72 hours after surgery. If the pain was not controlled by IV-PCA, additional IV analgesia was administered.
The cost of each procedure was obtained from the official hospital records of the accounting department in our institution. We compared the direct healthcare cost of each procedure, which includes the total hospital charge, supplies (cost of standard instruments and those unique to each procedure), manpower (1 day of work of the operating block), anesthesia cost, pharmacy, hospital stay, and 30-day readmission charge.
TNM staging was based on the Japanese Classification of Gastric Carcinoma, 3rd English edition [14]. Although numerous pathohistological classification systems have been established for gastric cancer, controversy remains as to which classification unifies a prognostic value [15]. However, the World Health Organization classification issued in 2010 is considered to be the most detailed among all pathohistological classification systems and therefore was used for this study.
All statistical calculations were performed using the IBM SPSS Statistics for Windows, ver. 21 (IBM Corp., Armonk, NY, USA). The demographic and clinicopathological characteristics were summarized using descriptive analysis, and all qualitative values are presented as mean and standard deviation unless expressed otherwise. The Student t-test or Mann-Whitney U-test and Pearson chi-square test were used to compare continuous and categorical variables, respectively, and survival data were estimated using the life table method. All P-values equal to 0.05 were considered significant.
The patients' demographic characteristics before and after the PSM are shown in Table 1. Before the PSM, there was a large sample size discrepancy between the 2 groups. These differences were stratified after matching. No significant differences were shown in age, sex, BMI, ASA PS classification, history of abdomen operation, tumor location, maximum tumor size in preoperative image study, and clinical stage in the matched groups.
Table 2 shows the operative outcomes between the solo SIDG and MLDG groups. All operations were performed in the R0, with no open conversion or transfusion. Mean operation time (120 ± 35.3 vs. 178.6 ± 53.4 minutes, P = 0.001) and EBL (24.6 ± 47.4 vs. 46.7 ± 66.5 mL, P = 0.004) were significantly lower in the solo SIDG group. No differences were observed in the number of retrieved lymph nodes and reconstruction methods. Among the reconstruction methods, uncut Roux-en-Y gastrojejunostomy was the most applied technique in both groups. With respect to the extent of lymph node dissection, the solo SIDG group had a significantly higher number of D2 lymphadenectomies, whereas the MLDG group had more D1+lymph node dissections (P = 0.001).
No significant differences in terms of postoperative morbidities were observed between the 2 groups. The early complication rates in solo SIDG and MLDG were 21.2% and 23.7%, respectively (P = 0.240), whereas the late complication rates in solo SIDG and MLDG were 7.1% and 11.1%, respectively (P = 0.672). Detailed information on complications is summarized in Table 3. No in-hospital mortality was observed in either group.
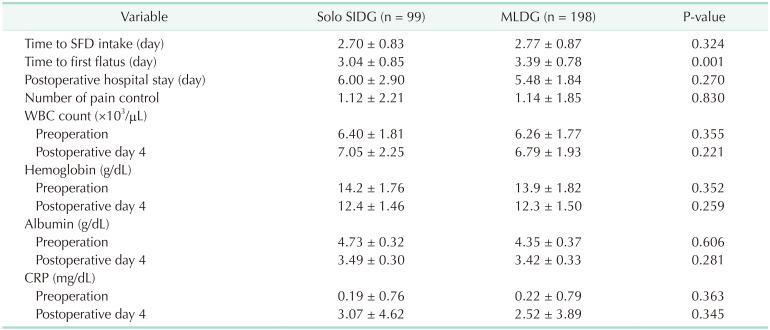
The postoperative recovery parameters including the time to SFD intake and first flatus, hospital stay, number of pain controls, and laboratory findings are summarized in Table 4. There was no significant difference in the time interval to SFD intake and hospital stay. However, postoperative recovery was faster in the solo SIDG group in terms of early initiation of flatus (3.04 ± 0.85 vs. 3.39 ± 0.78 days, P = 0.001). Because all patients had IV-PCA, the number of pain controls was relatively low in both groups. The WBC, albumin, hemoglobin, and CRP levels were not different between both groups in preoperative day and postoperative day 4.
To assess severity of postoperative morbidities, early complication cases were graded using the Clavien-Dindo classification. In the solo SIDG group, of 21 patients with complications, 11 (11.1%) had grade I, 5 (5.1%) had grade II, and 5 (5.1%) had grade III. In the MLDG group, 32 (16.2%) had grade I, 11 (5.6%) had grade II, and 4 (2.0%) had grade III. In the comparison of late complications, the severity was similar (P = 0.672).
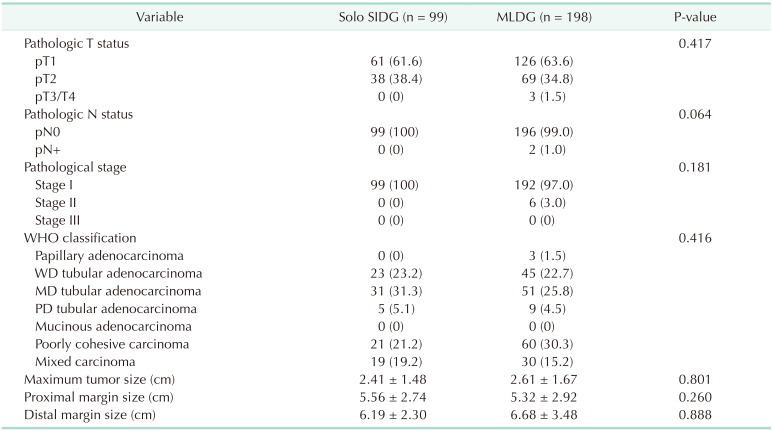
Table 5 shows the pathologic findings of all patients. Pathologic examinat ion of the specimens showed similar distribution between the 2 groups. Regarding the pathohistological classification, there was similar distribution between the 2 groups. Moderately differentiated tubular adenocarcinoma tissues were the most common subtypes in both groups. Specimens evaluated by a pathologist revealed that the mean tumor size was similar between the 2 groups. The mean proximal and distal margins of the specimens were larger than 5 cm.
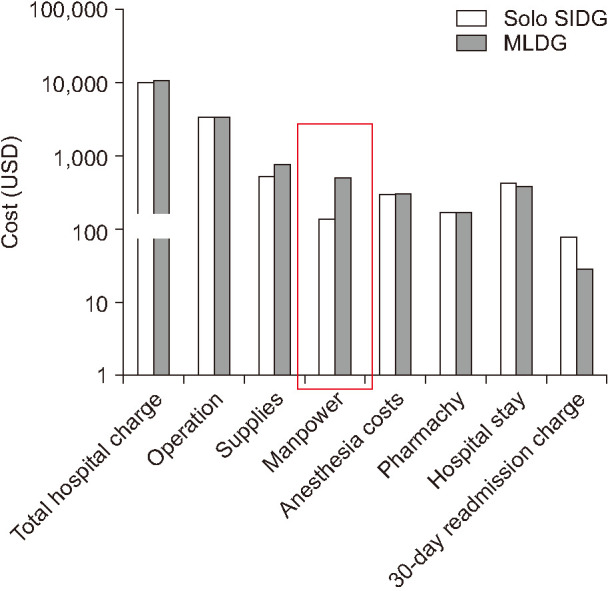
Fig. 1 shows the mean charge for the solo SIDG and MLDG groups. There were no significant differences in total hospital charge (solo SIDG:MLDG, 9,767.8:10,052.9 US dollars [USD]; P = 0.567), supplies (solo SIDG:MLDG, 558.7:794.1 USD; P = 0.070), anesthesia costs (solo SIDG:MLDG, 322.7:331.1 USD; P = 0.539), pharmacy (solo SIDG:MLDG 182.3:180.2 USD; P = 0.753), hospital stay (solo SIDG:MLDG, 445.8:407.1 USD; P = 0.431), and 30-day readmission charge (solo SIDG:MLDG, 82.2:32.5 USD; P = 0.150). However, in terms of manpower cost, solo SIDG showed a significantly lower cost than that of MLDG (solo SIDG:MLDG, 151.7:480.6 USD; P = 0.001).
This study was designed to compare the short-term outcomes, clinical experiences, and costs between solo SIDG and MLDG for gastric cancer in propensity-matched groups. It is the first report on the oncologic outcomes of solo laparoscopic gastrectomy. There was no difference in early complication (P = 0.240), late complication (P = 0.672), nor mortality (P > 0.999) between solo SIDG group and MLDG group during the follow-up period of 3 years. This result shows that solo SIDG is safe and feasible for EGC, with better operating time, EBL, and time to first flatus than MLDG, and has acceptable morbidity and short-term outcomes.
Single-port laparoscopic surgery for EGCs has several advantages; less invasiveness, excellent cosmetic results, reduced postoperative pain, and shortened recovery time [216]. Looking at the result of our postoperative hospital stay, there was no significant difference between solo SIDG and MLDG. This was probably due to using the same clinical pathway in both solo SIDG and MIDG patients. The usual clinical pathway for MIDG can readily be applied to solo SIDG without increasing postoperative complications.
When single-port surgery was introduced, only a few institutions performed single-port laparoscopic gastrectomy because of its technical difficulty. These operations pose a challenge to the operator, especially during meticulous dissection, due to constraints of the technique, mainly in instrument crossover and instruments clashing with the camera [1718]. With the development of techniques and instruments, SIDG could be expected to be the next step in “more” minimally invasive surgery [19]. Moreover, a recent study revealed that SIDG is oncologically safe and feasible for the treatment of gastric cancer [3].
When performing SIDG, a skilled camera operator is needed to show the surgical field. However, the number of experienced camera operators is insufficient, and they have a fixed working schedule, which limits the practice of SIDG. An inexperienced camera operator participating in SIDG will likely interfere with surgery because the camera will constantly clash with the instruments. With the implementation of solo SIDG, the number of required surgical assistants and types of needed instruments changes. Solo SIDG requires only an operator and a scrub nurse, whereas MLDG requires an operator, a first assistant, a camera operator, and a scrub nurse. With the implementation of solo SIDG, the number of required surgical assistants decreases, attributing to a significant cutting down of the labor cost for the surgery (P = 0.001).
Since solo surgery is performed solely by the surgeon, the most significant disadvantage is the potential lag time between the appearance of an emergency and surgical management [19]. In our institution, to assure patient safety, there were always assisting hands available when needed. There were no cases needing conversion surgery, though the surgeon was ready to apply an additional trocar or convert to open surgery. All bleedings during the operation were promptly controlled by the surgeon, with perhaps an easier environment for hemostasis with the scope-holder rather than with an inexperienced camera operator.
In terms of surgical outcomes of solo surgery, passive camera holders were compared with human camera operators in clinical and phantom experiments with no significant differences in the operation time [9]. Some reports described that solo surgery provides a more fixed and stable image under the direct control of the operator himself or herself [4]. Solo single-port laparoscopic gastrectomy lowered hospital costs without lengthening operation time [8]. According to a previous report, the mean operative time was similar between SIDG and MLDG [2]. In our study, mean operative time was significantly shorter in the solo SIDG group than in the MLDG group. Use of a mechanical camera holder, which eliminates miscommunication between the operator and assistant, might have contributed to shortening the operation time.
Based on the result of this study, the extent of lymph node dissection was greater (P = 0.001) but the EBL was significantly lower (P = 0.004) in the solo SIDG group. This signifies that when solo surgery is performed by an experienced surgeon, the operation can be done with better control and increased precision of action for dissection of layers between the organs. Although the extent of dissection was greater in solo SIDG group, there is no difference in lymph node yield (P = 0.823) between the solo SIDG group (57.1 ± 22.7) and MLDG group (60.6 ± 22.1). The reason for this might be due to the learning curve effect, having retrieved fewer lymph nodes in early stages of solo SIDG. Nevertheless, the total number of lymph nodes harvested is sufficient to prove that solo SIDG can provide optimal treatment and accurate staging.
Minimal manipulation during SIDG may reduce damage to visceral organs, resulting in attenuated inflammatory response [3]. CRP values can be used to monitor surgical trauma because it induces an almost immediate increase in circulating levels of interleukin 6 and subsequently of CRP [20]. Hence, we expected significantly lower postoperative parameters in the SIDG group. The results, however, showed that postoperative laboratory findings were not significantly different between the solo SIDG and MLDG groups. A study from Bulut et al. [21] also suggested that the trauma-induced inflammatory response of single-port operation may be similar to that of conventional laparoscopic surgery.
Further long-term follow-up results may be needed for accurate assessment of solo study, but the outcome of the short-term period is quite promising. There were 5 cases of grade III complications, and there was no significant difference with MLDG (P = 0.240). All patients were discharged with no lasting complications after proper management. There were 3 cases of postoperative mortality, 1 patient who underwent solo SIDG died of acute myocardial infarction due to ventricular septal defect 1 year after surgery, and 2 patients who underwent MLDG died of aspiration pneumonia and sepsis, but none of the deaths were related to cancer.
This study has several limitations. First, although the sample size was relatively large, this study was a retrospective propensity matching analysis, accompanying inevitable bias in the nature of study. Therefore, further evaluation of long-term outcomes and randomized controlled studies are required. Secondly, although our study favors superior results from SIDG, it is difficult to generalize the result since the surgery is done by a single experienced surgeon. From our previous study, it takes approximately 20 cases to become accustomed to solo-SIDG [12].
Despite these limitations, this study demonstrated that solo SIDG performed by an experienced laparoscopic surgeon is safe and feasible for EGC. Solo SIDG is expected to be a promising potential treatment method for EGC.
Notes
Author Contribution:
Conceptualization: BL, SIY, SHA, DJP, HHK.
Formal Analysis: BL, SIY, KL, YW, YTL, YSP.
Investigation: BL, SIY, KL, YW, SM, YTL, YSP.
Methodology: SM, YSP, SHA, DJP, HHK.
Project Administration: SHA, DJP, HHK.
Writing — Original Draft: BL, SIY, SM, YTL, YSP, SHA, DJP, HHK.
Writing — Review & Editing: BL, SIY, KL, YW, YSP, SHA, HHK.
References
1. Omori T, Tanaka K, Tori M, Ueshima S, Akamatsu H, Nishida T. Intracorporeal circular-stapled Billroth I anastomosis in single-incision laparoscopic distal gastrectomy. Surg Endosc. 2012; 26:1490–1494. PMID: 22044985.

2. Ahn SH, Son SY, Jung DH, Park DJ, Kim HH. Pure single-port laparoscopic distal gastrectomy for early gastric cancer: comparative study with multi-port laparoscopic distal gastrectomy. J Am Coll Surg. 2014; 219:933–943. PMID: 25256369.

3. Omori T, Fujiwara Y, Moon J, Sugimura K, Miyata H, Masuzawa T, et al. Comparison of single-incision and conventional multiport laparoscopic distal gastrectomy with D2 lymph node dissection for gastric cancer: a propensity score-matched analysis. Ann Surg Oncol. 2016; 23(Suppl 5):817–824. PMID: 27510844.

4. Kim SJ, Choi BJ, Jeong W, Lee SC. The feasibility of single-port laparoscopic appendectomy using a solo approach: a comparative study. Ann Surg Treat Res. 2016; 90:164–170. PMID: 26942160.

5. Velthuis S, van den Boezem PB, Lips DJ, Prins HA, Cuesta MA, Sietses C. Comparison of short-term surgical outcomes after single-incision laparoscopic versus multiport laparoscopic right colectomy: a two-center, prospective case-controlled study of 100 patients. Dig Surg. 2012; 29:477–483. PMID: 23364285.

6. Lurje G, Raptis DA, Steinemann DC, Amygdalos I, Kambakamba P, Petrowsky H, et al. Cosmesis and body image in patients undergoing single-port versus conventional laparoscopic cholecystectomy: a multicenter double-blinded randomized controlled trial (SPOCC-trial). Ann Surg. 2015; 262:728–735. PMID: 26583659.
7. Podda M, Saba A, Porru F, Pisanu A. Systematic review with meta-analysis of studies comparing single-incision laparoscopic colectomy and multiport laparoscopic colectomy. Surg Endosc. 2016; 30:4697–4720. PMID: 26905578.

8. Jaspers JE, Breedveld P, Herder JL, Grimbergen CA. Camera and instrument holders and their clinical value in minimally invasive surgery. Surg Laparosc Endosc Percutan Tech. 2004; 14:145–152. PMID: 15471021.

9. Arezzo A, Ulmer F, Weiss O, Schurr MO, Hamad M, Buess GF. Experimental trial on solo surgery for minimally invasive therapy: comparison of different systems in a phantom model. Surg Endosc. 2000; 14:955–959. PMID: 11080411.
10. Caliendo M, Kopeinig S. Some practical guidance for the implementation of propensity score matching. J Econ Surv. 2008; 22:31–72.

11. Daabiss M. American Society of Anaesthesiologists physical status classification. Indian J Anaesth. 2011; 55:111–115. PMID: 21712864.

12. Kang SH, Cho YS, Min SH, Park YS, Ahn SH, Park DJ, et al. Early experience and learning curve of solo single-incision distal gastrectomy for gastric cancer: a review of consecutive 100 cases. Surg Endosc. 2019; 33:3412–3418. PMID: 30604257.

13. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6,336 patients and results of a survey. Ann Surg. 2004; 240:205–213. PMID: 15273542.
14. Japanese Gastric Cancer Association. Japanese classi fication of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011; 14:101–112. PMID: 21573743.
15. Berlth F, Bollschweiler E, Drebber U, Hoelscher AH, Moenig S. Pathohistological classification systems in gastric cancer: diagnostic relevance and prognostic value. World J Gastroenterol. 2014; 20:5679–5684. PMID: 24914328.

16. Park DJ, Lee JH, Ahn SH, Eng AK, Kim HH. Single-port laparoscopic distal gastrectomy with D1+β lymph node dissection for gastric cancers: report of 2 cases. Surg Laparosc Endosc Percutan Tech. 2012; 22:e214–e216. PMID: 22874704.
17. Ozdemir BA, Thomas RL, Soon Y. Single-port laparoscopic subtotal gastrectomy with DIα lymphadenectomy. Surg Innov. 2011; 18:NP1–NP4.

18. Suh YS, Lee HJ, Yang HK. Single incision gastrectomy for gastric cancer. Transl Gastroenterol Hepatol. 2016; 1:41. PMID: 28138608.

19. Kim SJ, Lee SC. Technical and instrumental prerequisites for single-port laparoscopic solo surgery: state of art. World J Gastroenterol. 2015; 21:4440–4446. PMID: 25914453.

20. Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium. Swerdlow DI, Holmes MV, Kuchenbaecker KB, Engmann JE, Shah T, et al. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomization analysis. Lancet. 2012; 379:1214–1224. PMID: 22421340.
21. Bulut O, Aslak KK, Levic K, Nielsen CB, Rømer E, Sørensen S, et al. A randomized pilot study on single-port versus conventional laparoscopic rectal surgery: effects on postoperative pain and the stress response to surgery. Tech Coloproctol. 2015; 19:11–22. PMID: 25380743.

Fig. 1
Mean charge for the cost between solo single-incision laparoscopic distal gastrectomy (SIDG) vs. multiport laparoscopic distal gastrectomy (MLDG) groups. USD, US dollar.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download