Abstract
Purpose
Laparoscopic distal pancreatectomy (LDP) is widely performed but its efficacy and safety are not established for malignant lesions. This study was aimed to compare outcomes of LDP and open distal pancreatectomy (ODP) in pancreatic ductal adenocarcinoma (PDAC).
Methods
Patients who underwent distal pancreatectomy for PDAC between 2009 and 2017 were enrolled. The preoperative clinical stage was evaluated and propensity score matching (PSM) was performed using age, sex, The American Joint Committee on Cancer 8th clinical T stage, and other organ involvement.
Results
In 186 patients enrolled, 35 (18.8%) received LDP. The ODP group showed larger tumor size and frequent involvement of other organs in preoperative images. However, after PSM, these differences were balanced. R0 resection (90.5% vs. 94.3%, P = 0.730), harvested lymph nodes (14.3 vs. 12.6, P = 0.380) and pathologic T stage (P = 0.474) were comparable between ODP and LDP groups, respectively. LDP demonstrated shorter operation time, less postoperative pain, and shorter hospitalization (14.4 days vs. 11.1 days, P = 0.026). In terms of long-term oncologic outcomes, median overall survival (32 months vs. 28 months, P = 0.724) and disease-free survival (18 months vs. 19 months, P = 0.926) were comparable.
Laparoscopic surgery has been proven beneficial in various malignant lesions of the digestive system. For gastric cancer, laparoscopic gastrectomy has been widely used and has demonstrated better outcomes compared with conventional open surgery [12]. The advantages of laparoscopic surgery for colorectal cancer have also been established by several randomized controlled trials [3456]. In contrast, there is no high-level evidence for the efficacy and safety of laparoscopic surgery in pancreatic cancer, and its feasibility also remains controversial.
Despite these controversies regarding laparoscopic pancreatectomy for pancreatic cancer, the use of laparoscopic pancreatectomy has been expanded constantly. Laparoscopic pancreaticoduodenectomy is generally performed by several experts due to its complexity [78]. Compared to laparoscopic pancreaticoduodenectomy, laparoscopic distal pancreatectomy (LDP) is widely performed because it does not require complicated reconstruction.
The efficacy and safety of LDP for benign or borderline diseases were proven by a randomized controlled trial (RCT) that compared it with open distal pancreatectomy (ODP) [9]. The authors reported a shorter time to functional recovery and a better quality of life after LDP. In contrast, in the case of malignant pancreatic lesions, there are no RCTs on the long-term oncologic outcomes; there are only retrospective studies investigating the short-term and long-term outcomes of LDP and ODP [101112131415]. In the case of retrospective studies, the treatment-selection bias was inevitable which prevented meaningful comparisons. Therefore, statistical methods to minimize the impact of treatment selection must be used.
Therefore, the purpose of this study was to evaluate the safety and feasibility of LDP for pancreatic ductal adenocarcinoma (PDAC) by comparing perioperative short-term outcomes and oncologic long-term outcomes using the propensity score matching (PSM) method.
This study was a retrospective cohort study with prospectively collected data. Patients who underwent distal pancreatectomy (DP) at Seoul National University Hospital (Seoul, Korea) between January 2009 and December 2017 were reviewed. Patients who underwent curative-intent surgery with pathologically confirmed PDAC were included in the current study. Patients with distant metastasis, double primary lesions, or those receiving neoadjuvant treatment for improved tumor resectability were excluded. LDP was performed in patients with a lesion which was localized in the pancreatic parenchyma without pancreatitis and involvement of adjacent organ such as colon and stomach on preoperative CT images. In addition, there was no evidence for invasion of major vessels including celiac artery, common hepatic artery, and superior mesenteric artery. Body mass index (BMI) was not restricted in selecting patients to receive LDP. None of them received neoadjuvant therapy before surgery. Open conversion cases, for any reason, were included in the ODP group. This study was approved by the Institutional Review Board of Seoul National University Hospital (No. SNUH-2007-095-1141) with a waiver for informed consent.
The baseline characteristics were investigated including age, sex, BMI, American Society of Anesthesiologists (ASA) physical status, and underlying disease. All patients received CT or MRI before surgery to determine the preoperative clinical stage. Images of all cases were reviewed for tumor size and involvement of other organs or splenic vessels. Tumor markers including CA 19-9 and CEA were also checked before surgery.
Perioperative clinical outcomes including operation-related data, pathologic data, and clinical outcomes were investigated. Postoperative pain was estimated according to the numeric rating scale (NRS) on postoperative day (POD) 1 and 3. Duration of postoperative hospital stay and interval from operation to adjuvant treatment were investigated. To evaluate long-term oncologic outcomes, median overall survival (OS), median disease-free survival (DFS), 5-year OS rate, and 5-year DFS rate were investigated.
During the dissection around the distal pancreas, lymph nodes (LNs) in the inferior pancreatic, splenic arterial, gastrosplenic, and splenic hilar area were routinely dissected. Pancreas transection was performed on the superior mesenteric vein with as endoscopic triple-line linear stapler (Endo-GIA, Autosuture Corp., Norwalk, CT, USA; Echelone-Flex, Ethicon, Somerville, NJ, USA). In ODP patients, we used the same stapling method or sewed by hand. If the patient was suspected to have or was diagnosed with PDAC before surgery, spleen preservation was not attempted. Combined organ resection was defined as resection of other organs such as the adrenal gland or colon, excluding the spleen.
All patients were managed with similar postoperative treatment. CT was performed for every patient POD 4 to detect clinically relevant postoperative pancreatic fistula (CR-POPF) and other complications. The amylase level was also checked using blood serum and intraabdominal fluid from drainage. The CR-POPF grade was defined according to the International Study Group on Pancreas Surgery criteria [16].
Continuous variables were expressed as the mean and standard deviation (SD) and were analyzed with the Student t-test or Mann-Whitney U-test as appropriate. Categorical variables were compared using the chi-squared test or Fisher exact test.
Matching between the ODP and LDP patients was performed by estimating a propensity score for each patient and matching the patients from each group in a 3:1 ratio. To estimate the propensity score, a logistic regression model using 4 covariates was performed. Each patient who underwent LDP was matched to a patient who underwent ODP by using nearest neighbor matching at a ratio of 3:1 within a specified caliper width. This matching was performed without replacement and by using a caliper width of 0.2 SDs of the logit of the estimated propensity score.
Survival analysis was performed using the Kaplan-Meier estimated survival. Statistical significance was defined as P < 0.05. To determine the independent prognostic factors for survival outcomes, multivariate analysis was performed. A Cox proportional hazards model was used for multivariable analysis. All statistical calculations were made using the IBM SPSS Statistics ver. 25.0 (IBM Corp., Armonk, NY, USA).
A total of 233 patients diagnosed with PDAC underwent DP between January 2009 and December 2017. Among these patients, 25 patients with distant metastasis or double primary tumor and 22 patients who received neoadjuvant therapy were excluded. Consequently, 186 patients were enrolled in this study, of whom 35 patients (18.8%) received LDP and 151 patients (81.2%) received ODP. There were 2 open conversion cases, the first patient was due to bleeding and the second patient was due to superior mesenteric vein involvement.
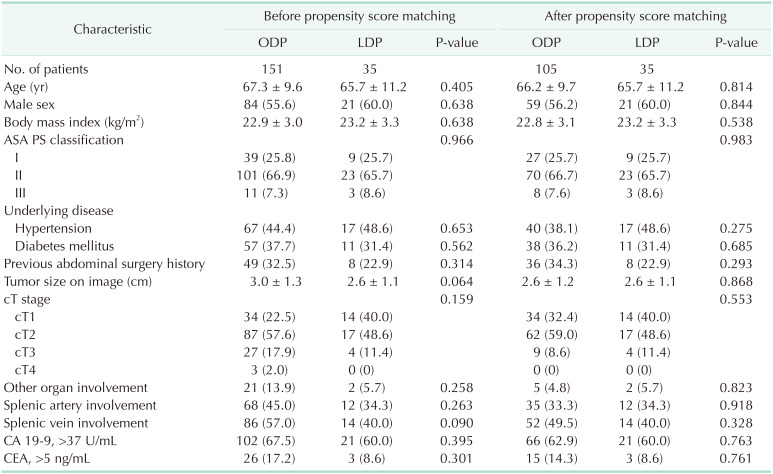
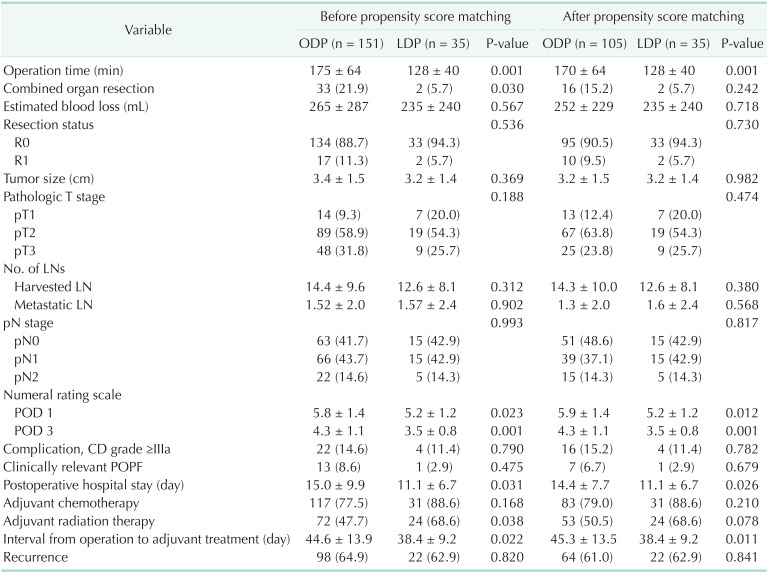
The baseline characteristics and preoperative clinical findings of each group are shown in Table 1. The demographics including age, sex, BMI, ASA physical status, underlying disease, and previous abdominal operation history were comparable between the ODP and LDP groups.
In preoperative evaluation, patients in the ODP group tended to show larger tumor size on CT images compared with patients in the LDP group (3.0 cm vs. 2.6 cm, respectively; P = 0.064). There were also differences in the composition of clinical T (cT) stage and invasion into other organs on preoperative imaging between the ODP and LDP groups, which were not statistically significant. The number of patients who demonstrated higher than the normal levels of tumor markers, such as CA 19-9 and CEA, was comparable between the ODP and LDP groups.
To make the composition of patients comparable, we performed PSM with 4 variables which revealed differences between the ODP and LDP groups, including age, sex, cT stage, and other organ involvement. After PSM, differences in preoperative clinical evaluation between the ODP and LDP groups were balanced. Tumor size and involvement of other organs became comparable on preoperative imaging between the ODP and LDP groups.
Perioperative short-term clinical outcomes including pathologic findings are shown in Table 2. Before PSM, LDP demonstrated shorter operation time and less combined organ resection compared with ODP. The mean estimated blood loss was comparable between the ODP and LDP groups. After PSM, the mean operation time of LDP was shorter than that of ODP (170 minutes vs. 128 minutes, respectively; P = 0.001).
The pathologic findings including R0 resection rate (90.5% vs. 94.3%, respectively; P = 0.730), tumor size, and the mean number of harvested and metastatic LNs were comparable between the ODP and LDP groups. There were also no significant differences in the pathologic T (pT) and pathologic N stage between groups.
For pain after surgery, the mean NRS of the ODP was higher than that of LDP patients on POD 1 and POD 3. During the hospital stay, 22 patients (14.6%) in the ODP group and 4 patients (11.4%) in the LDP group had complications. The CR-POPF rates were comparable between the ODP and LDP groups (8.6% vs. 2.9%, P = 0.475). Patients in the LDP group demonstrated a shorter duration of hospital stay than those in the ODP group (15.0 days vs. 11.1 days, respectively; P = 0.031). Also, interval time between surgery and adjuvant treatment was shorter in LDP group (44.6 days vs. 38.4 days, respectively; P = 0.022). After PSM, patients in the LDP group still demonstrated less postoperative pain on POD 1 and POD 3 and a shorter duration of postoperative hospital stay and interval from surgery to adjuvant treatment.
Regarding the entire cohort, the median OS was 30 months and the 5-year OS rate was 31.1%. The median DFS was 16 months and the 5-year DFS rate was 23.8%. Before PSM, survival outcomes of the ODP group were comparable with those of the LDP group (Fig. 1). The median OS in the ODP and LDP group was 30 and 28 months, respectively (P = 0.879), and the median DFS was 16 and 19 months, respectively (P = 0.672). After PSM, there was no difference in survival outcomes between the ODP and LDP groups (Fig. 2). The median OS in the ODP and LDP group was 32 and 28 months, respectively (P = 0.724), and the median DFS was 18 and 19 months, respectively (P = 0.926). The OS and DFS of a patient who underwent open conversion due to major vessel invasion were 12 months. The OS and DFS of the other patient who underwent open conversion due to intraabdominal bleeding were 46 and 18 months. In addition, as we performed survival analysis including 2 open conversion cases into LDP group, the median OS and DFS was 28 months and 18 months, respectively. These results were also comparable with ODP group.
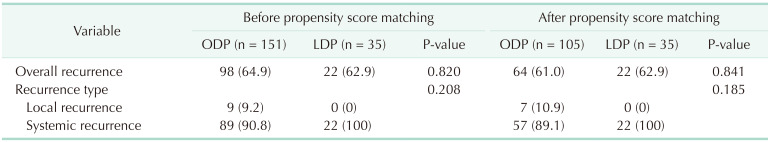
The recurrence rate and recurrence patterns are shown in Table 3. After PSM, there was no significant difference in the overall recurrence rate, and the type of recurrence between the ODP and LDP groups (58.9% vs. 62.9%, respectively; P = 0.670).
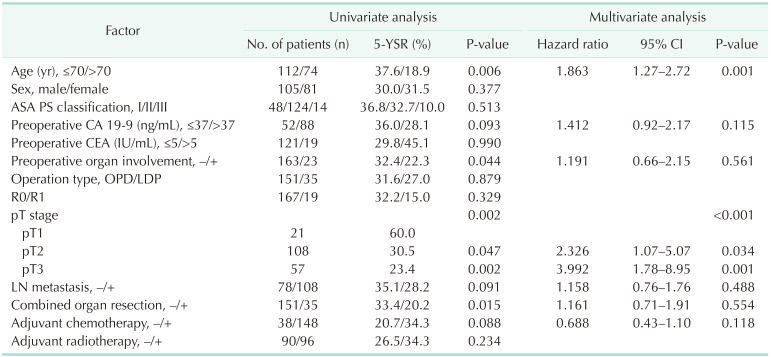
Further analysis was performed to identify the factors that affect the survival outcomes. In univariate and multivariate analysis, the age (hazard ratio [HR], 1.818; P = 0.002) and pT stage (T2: HR, 2.326; P = 0.034; T3: HR, 3.992; P = 0.001) were identified as prognostic factors for 5-year OS rate in patients who received DP for PDAC (Table 4). However, the type of surgery had no impact on survival outcomes (P = 0.879).
LDP has been widely performed by pancreatic surgeons because it does not require reconstruction and is technically easier than pancreaticoduodenectomy. Although there is no oncologic high-level evidence to support the benefits of laparoscopic surgery in pancreatic cancer, pancreatic surgeons have made a continuous effort to establish the advantages of LDP by comparing surgical outcomes with ODP. However, meaningful comparisons in previous studies have been prevented by the small cohort sizes and the treatment-selection bias of retrospective studies. Therefore, the current study was designed to minimize selection bias by using PSM to compare perioperative short-term and oncologic long-term outcomes of ODP and LDP in matched cohorts of PDAC patients.
Treatment-selection bias occurred before surgery because the surgeon decided which patients would undergo ODP or LDP by preoperative images. Patients who showed larger tumor size or invasion into other organs tended to undergo ODP, rather than LDP. Although RCT is well known to be the most powerful method for minimizing the impact of selection bias, the small number of cases and the disastrous prognosis of pancreatic cancer make it difficult to design RCT. Therefore, we performed PSM with variables including age, sex, cT stage, and other organ involvement evaluated by preoperative images. After PSM, differences in preoperative findings between ODP and LDP were balanced.
In early laparoscopic surgery, pancreatic surgeons were concerned about oncologic safety in terms of R0 resection and adequate lymphadenectomy, which were known to be important prognostic factors of PDAC [17181920]. It is more difficult to obtain a negative resection margin and adequate lymphadenectomy in pancreatic cancer compared with other gastrointestinal cancers due to its anatomical position, major adjacent vessels, and extensive surgical range. However, surgeons have constantly striven to prove the oncologic safety of LDP against ODP in PDAC patients. Zhang et al. [21] investigated 98 patients with PDAC, in which 22 patients underwent LDP. They reported 87% vs. 91% R0 resection rates, and mean number of harvested LNs in ODP and LDP patients, respectively. In our study, R0 resection rates and the mean number of harvested LNs were also comparable between the ODP and LDP groups. Consequently, LDP is an appropriate method for obtaining oncological clearance in terms of the negative resection margin and the sufficient number of harvested LNs.
In propensity-matched patients, those patients who received LDP demonstrated less pain and shorter hospitalization after surgery. Many studies also reported early recovery and shorter hospital stay following LDP compared to ODP in PDAC patients [222324]. These were results of minimal manipulation of organs during surgery and smaller surgical wounds due to the laparoscopic approach. These improved short-term outcomes could lead to improved oncologic long-term outcomes. Early recovery after surgery facilitates administration of proper adjuvant chemotherapy, which has significant survival benefits for patients with pancreatic cancer [2526]. Neoptolemos et al. [27] reported that the 5-year survival rate was 21% among 147 resected pancreatic cancer patients who received chemotherapy and 8% among 142 patients who did not receive chemotherapy (P = 0.009). In our study, LDP group demonstrated shorter interval from surgery to adjuvant treatment. Therefore, early recovery after LDP could theoretically be related to better survival outcomes.
The comparable survival outcomes between ODP and LDP patients are consistent with other reports in the literature. Shin et al. [24] from Asan Medical Center in Korea reported that 1-, 2-, and 5-year OS rates were 87.6%, 64.3%, and 32.5% in 70 LDP patients which were comparable with ODP group (P = 0.250). Also, van Hilst et al. [23] reported median OS was 31 months in 340 LDP patients and 28 months in 856 ODP patients. These results were due to similar pathologic findings with the LDP and ODP group, such as R0 resection rate, tumor size, and the number of metastatic LNs. Furthermore, a similar extent and procedure of surgery between the ODP and LDP groups could lead to comparable survival outcomes. Although most studies reported comparable survival outcomes between these 2 groups, Sulpice et al. [28] reported improved survival of LDP patients over ODP patients. They reported that median survival was 62.5 months for 347 LDP patients and 36.7 months for 2,406 ODP patients. They explained that these results were due to selection bias or a lower incidence of splenectomy and blood transfusion of LDP patients. However, there were no statistical methods to reduce selection bias between the 2 groups. In our study, a patient age greater than 70 years, and the pT stage of PDAC were also independently associated with OS. Kooby et al. [10] also reported risk factors for patients with PDAC including advanced patient age, larger tumor size, LN and margin positive resections, and absence of adjuvant therapy.
The present study does have some limitations. First, selection bias could have occurred since the majority of patients in the LDP group may have been selected due to having a small tumor without invasion into other organs. Although PSM was performed, it was impossible to completely eliminate the impact of treatment selection bias. Second, as a limitation of the retrospective study, surgeons performed LDP for more advanced cases in the latter part of the study period. However, most of the LDP was performed according to the inclusion criteria. Last, LDP tended to be performed more recently than ODP. In 2009, the first case of LDP was performed and the ratio of LDP over ODP was 7.1%. The ratio of LDP over ODP increased gradually, reaching 17.1% in 2016 and 25.7% in 2017. These trends potentially resulted in bias due to improvement of surgical technique and perioperative management, such as chemotherapy and radiation therapy.
In conclusion, patients in the LDP group showed better perioperative short-term outcomes in terms of the operation time, postoperative pain, and hospital stay compared with the ODP group. In terms of oncologic long-term outcomes, median OS and DFS were comparable. Therefore, LDP can be used as a safe and feasible surgery for PDAC patients.
Notes
References
1. Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, Kim W, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report. A phase III multicenter, prospective, randomized trial (KLASS Trial). Ann Surg. 2010; 251:417–420. PMID: 20160637.
2. Viñuela EF, Gonen M, Brennan MF, Coit DG, Strong VE. Laparoscopic versus open distal gastrectomy for gastric cancer: a meta-analysis of randomized controlled trials and high-quality nonrandomized studies. Ann Surg. 2012; 255:446–456. PMID: 22330034.
3. Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MH, de Lange-de Klerk ES, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med. 2015; 372:1324–1332. PMID: 25830422.

4. Hasegawa H, Kabeshima Y, Watanabe M, Yamamoto S, Kitajima M. Randomized controlled trial of laparoscopic versus open colectomy for advanced colorectal cancer. Surg Endosc. 2003; 17:636–640. PMID: 12574925.

5. Kennedy RH, Francis EA, Wharton R, Blazeby JM, Quirke P, West NP, et al. Multicenter randomized controlled trial of conventional versus laparoscopic surgery for colorectal cancer within an enhanced recovery programme: EnROL. J Clin Oncol. 2014; 32:1804–1811. PMID: 24799480.

6. van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013; 14:210–218. PMID: 23395398.

7. Croome KP, Farnell MB, Que FG, Reid-Lombardo KM, Truty MJ, Nagorney DM, et al. Total laparoscopic pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: oncologic advantages over open approaches? Ann Surg. 2014; 260:633–638. PMID: 25203880.
8. Song KB, Kim SC, Hwang DW, Lee JH, Lee DJ, Lee JW, et al. Matched case-control analysis comparing laparoscopic and open pylorus-preserving pancreaticoduodenectomy in patients with periampullary tumors. Ann Surg. 2015; 262:146–155. PMID: 25563866.

9. de Rooij T, van Hilst J, van Santvoort H, Boerma D, van den Boezem P, Daams F, et al. Minimally invasive versus open distal pancreatectomy (LEOPARD): a multicenter patient-blinded randomized controlled trial. Ann Surg. 2019; 269:2–9. PMID: 30080726.
10. Kooby DA, Hawkins WG, Schmidt CM, Weber SM, Bentrem DJ, Gillespie TW, et al. A multicenter analysis of distal pancreatectomy for adenocarcinoma: is laparoscopic resection appropriate? J Am Coll Surg. 2010; 210:779–785. PMID: 20421049.

11. Rehman S, John SK, Lochan R, Jaques BC, Manas DM, Charnley RM, et al. Oncological feasibility of laparoscopic distal pancreatectomy for adenocarcinoma: a single-institution comparative study. World J Surg. 2014; 38:476–483. PMID: 24081543.

12. Zhang M, Fang R, Mou Y, Chen R, Xu X, Zhang R, et al. LDP vs ODP for pancreatic adenocarcinoma: a case matched study from a single-institution. BMC Gastroenterol. 2015; 15:182. PMID: 26695506.

13. Kim EY, Hong TH. Initial experience with laparoscopic radical antegrade modular pancreatosplenectomy for left-sided pancreatic cancer in a single institution: technical aspects and oncological outcomes. BMC Surg. 2017; 17:2. PMID: 28061895.

14. Kim S, Yoon YS, Han HS, Cho JY. Laparoscopic subtotal pancreatectomy with radical antegrade modular pancreatosplenectomy for left-sided pancreatic cancer. Surg Oncol. 2019; 28:150. PMID: 30851891.

15. Lee SH, Kang CM, Hwang HK, Choi SH, Lee WJ, Chi HS. Minimally invasive RAMPS in well-selected left-sided pancreatic cancer within Yonsei criteria: long-term (>median 3 years) oncologic outcomes. Surg Endosc. 2014; 28:2848–2855. PMID: 24853839.
16. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017; 161:584–591. PMID: 28040257.
17. Geer RJ, Brennan MF. Prognost ic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg. 1993; 165:68–72. PMID: 8380315.
18. Ozaki H, Kishi K. Lymph node dissection in radical resection for carcinoma of the head of the pancreas and periampullary region. Jpn J Clin Oncol. 1983; 13:371–377. PMID: 6887557.
19. Fatima J, Schnelldorfer T, Barton J, Wood CM, Wiste HJ, Smyrk TC, et al. Pancreatoduodenectomy for ductal adenocarcinoma: implications of positive margin on survival. Arch Surg. 2010; 145:167–172. PMID: 20157085.
20. Moon HJ, An JY, Heo JS, Choi SH, Joh JW, Kim YI. Predicting survival after surgical resection for pancreatic ductal adenocarcinoma. Pancreas. 2006; 32:37–43. PMID: 16340742.

21. Zhang AB, Wang Y, Hu C, Shen Y, Zheng SS. Laparoscopic versus open distal pancreatectomy for pancreatic ductal adenocarcinoma: a single-center experience. J Zhejiang Univ Sci B. 2017; 18:532–538. PMID: 28585429.

22. Bauman MD, Becerra DG, Kilbane EM, Zyromski NJ, Schmidt CM, Pitt HA, et al. Laparoscopic distal pancreatectomy for pancreatic cancer is safe and effective. Surg Endosc. 2018; 32:53–61. PMID: 28643065.

23. van Hilst J, de Rooij T, Klompmaker S, Rawashdeh M, Aleotti F, Al-Sarireh B, et al. Minimally invasive versus open distal pancreatectomy for ductal adenocarcinoma (DIPLOMA): a pan-European propensity score matched study. Ann Surg. 2019; 269:10–17. PMID: 29099399.
24. Shin SH, Kim SC, Song KB, Hwang DW, Lee JH, Lee D, et al. A comparative study of laparoscopic vs. open distal pancreatectomy for left-sided ductal adenocarcinoma: a propensity scorematched analysis. J Am Coll Surg. 2015; 220:177–185. PMID: 25529901.

25. Heinemann V, Boeck S, Hinke A, Labianca R, Louvet C. Meta-analysis of randomized trials: evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer. 2008; 8:82. PMID: 18373843.

26. Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013; 310:1473–1481. PMID: 24104372.
27. Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004; 350:1200–1210. PMID: 15028824.

28. Sulpice L, Farges O, Goutte N, Bendersky N, Dokmak S, Sauvanet A, et al. Laparoscopic distal pancreatectomy for pancreatic ductal adenocarcinoma: time for a randomized controlled trial? Results of an all-inclusive national observational study. Ann Surg. 2015; 262:868–873. PMID: 26583678.
Fig. 1
The Kaplan-Meier survival curve of open (ODP) and laparoscopic distal pancreatectomy (LDP) before propensity score matching (PSM). Before PSM, (A) overall survival and (B) disease-free survival between ODP and LDP patients were comparable.
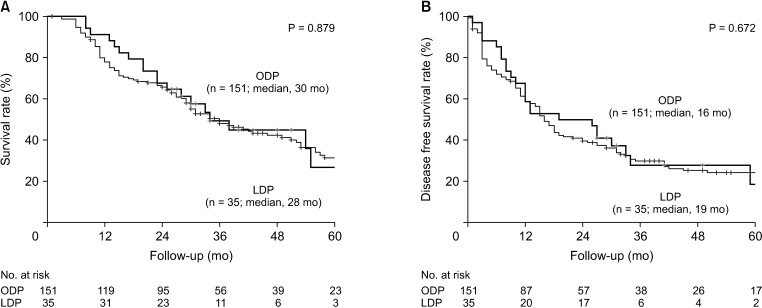
Fig. 2
The Kaplan-Meier survival curve of open (ODP) and laparoscopic distal pancreatectomy (LDP) after propensity score matching (PSM). After PSM, (A) overall survival and (B) disease-free survival between ODP and LDP patients were comparable.
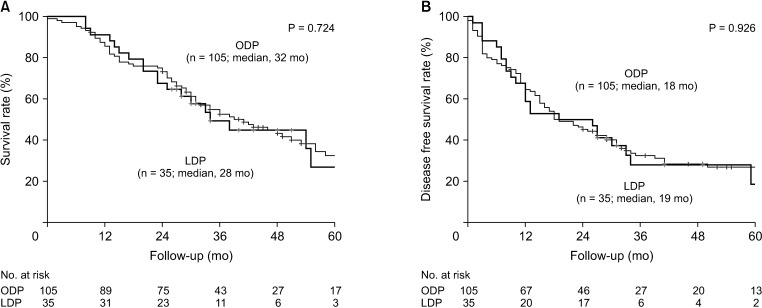
Table 3
Recurrence patterns between open and laparoscopic distal pancreatectomy in resected pancreas body and tail cancer




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download