INTRODUCTION
Pancreaticoduodenectomy is one of the most technically difficult abdominal operations, comprised of extensive dissection, resection, and reconstruction of the digestive system [
1]. In the past, surgeons could only perform an open pancreaticoduodenectomy through a long abdominal incision. Since the first laparoscopic pancreaticoduodenectomy (LPD) in 1994, minimally invasive approaches to pancreatic head lesions have been established and if the operation was completely performed intracorporeally, the technique was referred to as a totally LPD (TLPD) [
2]. The benefits of minimally invasive surgery are reduced analgesic requirements, reduced wound-related complications, shorter length of hospital stay, and faster return to normal daily activities [
3]. Laparoscopic surgery, however, has technical limitations, which include restricted degrees of motion of the laparoscopic instruments, reductions in hand-eye coordination, and impairments in the depth perception by the 2-dimensional image [
4]. Most importantly, the long, rigid laparoscopic instruments result in the ready transmission and exaggeration of tiny movements from the surgeon, making delicate procedures, particularly fine anastomoses, difficult. In particular, these shortcomings were remarkable when pancreatic reconstruction, called the Achilles' heel of pancreaticoduodenectomy, was performed [
5].
In 2001, robot-assisted LPD (RLPD) was reported by Giulianotti et al. [
6] for the first time. Since then, surgical robotic systems have been gradually applied in the field of pancreatic surgery. These systems exhibit more advantages compared to laparoscopic surgery, including articulation of the instruments with almost 540° of motion, providing more precise manipulation by elimination of surgeon tremors, and improving visualization in 3 dimensions [
7]. However, there are still some barriers to the implementation of robotic assistance in pancreatic surgery because of the location of the pancreas and the complex adjacent vascular structures. As surgical techniques need to respond to new technological advancements, it is critical to determine the safety, feasibility, and efficacy of these novel approaches [
8].
Few studies have reported the comparative surgical results of RLPD and TLPD. In this study, we analyzed the surgical effects of adopting a robotic surgical system in the long process of minimally invasive pancreaticoduodenectomy (MIPD) at a high-volume pancreatic center and examined the effectiveness and stability of RLPD.
Go to :

METHODS
From January 2016 and May 2020, a total of 101 patients underwent a MIPD in the Department of Hepatobiliary and Pancreatic Surgery at The Catholic University of Korea, Seoul St. Mary's Hospital in Seoul, Korea. Patients with tumors confined to the pancreatic head or periampullary region without vascular invasion, and the American Society of Anesthesiologists (ASA) physical status (PS) classification of <III were the subject of MIPD.
Among the patients who underwent TLPD or RLPD, this study was conducted only on the patients who had duct-to-mucosa pancreatojejunostomy anastomosis. Nine patients who underwent pancreatic reconstruction by mini-laparotomy or other anastomosis methods like dunking or pancreaticogastrostomy or who had unplanned conversion to open during surgery were excluded from the study. Finally, a total of 92 cases of RLPD and TLPD were analyzed to determine the perioperative and surgical outcomes between RLPD and TLPD.
This study was conducted after approval by the Institutional Review Board (KC20RISI0574). This study was conducted after approval by the Institutional Review Board (KC20RISI0574) with a waiver for informed consent.
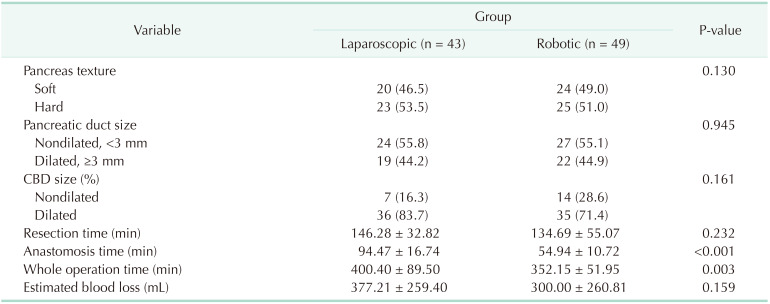
Data collection
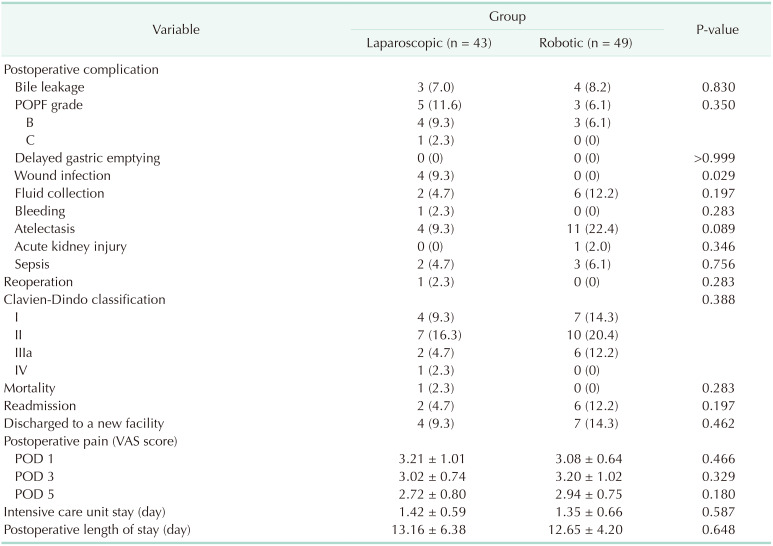
We collected data from electronic medical records, including several variables related to patient characteristics, operative variables, and surgical outcomes. The patients' demographic data were reviewed, including past medical history, sex, age, ASA PS classification, body mass index (BMI), and Charlson comorbidity index. Data on the whole operation time (WOT), anastomosis time (AT) in hepaticojejunostomy and pancreaticojejunostomy, texture of the pancreas, size of the pancreatic duct, estimated blood loss, and blood transfusions were collected as operative variables. The surgical outcomes included the largest tumor size, resection margin status, the number of harvested lymph nodes, pathologic results, postoperative complications including postoperative pancreatic fistula (POPF), length of hospital stay, readmission rate within 90 days after discharge from the hospital, and the 90-day mortality rate.
Definitions
We measured the WOT and the AT, which was done by laparoscopy-sewing (TLPD) or robot-sewing (RLPD), through a complete video review and analysis performed by 2 investigators who were not related to the operation. The resection time was defined as the time from opening the lesser sac to uncinate process dissection. The duodenojejunostomy time was not measured in the AT because it was hand-sewn in the extracorporeal site in both groups. The texture of the pancreas was subjectively classified as either soft or hard. The caliber of the main pancreatic duct was measured by the inner diameter of the internal self-removed Silastic tube, which was placed according to the pancreatic duct size during pancreas anastomosis and divided into the nondilated duct (<3 mm) and dilated duct (≥3 mm) [
9]. We also divided the diameter of the common bile duct (CBD) into the dilated CBD (defined as ≥1 cm in diameter) and nondilated CBD according to the radiologic image [
10].
The postoperative complications were graded using the Clavien-Dindo classification. The major complications were defined as events requiring surgical, endoscopic, or radiological intervention (Clavien-Dindo classification ≥ III) [
11]. The grading for POPFs was based on the International Study Group of Pancreatic Fistula (biochemical leak, B, and C) [
12].
Surgical procedures
Resection stage
In the surgical procedure, the TLPD and RLPD were basically performed in the same fashion according to patient position, trocar insertion site, and dissection sequence, and the same energy device was used. The resection began with the patient in the reverse Trendelenburg position, and a 10-mm trocar was inserted into the umbilical site for the laparoscope, two 12-mm trocars were inserted into the right and left hypogastrium, and two 5-mm trocars were inserted into the right and left subcostal location. Energy devices such as the THUNDERBEAT (Olympus Medical Systems Corp., Tokyo, Japan) and LigaSure (ValleyLab Inc., Boulder, CO, USA) were used in both groups during the entire operation. Dissection of the lymph nodes followed the recommendation of the International Study Group for Pancreatic Surgery (ISGPS) in the case of malignant tumors [
13]. Gastric and pyloric nodes and nodes to the right of the hepatoduodenal ligament were harvested
en bloc. The anterior and posterior pancreaticoduodenal nodes, nodes to the right of the superior mesenteric artery, and nodes anterior to the common hepatic artery were also harvested with the dissected head of the pancreas. After the completion of the resection stage, about 1 cm of the pancreatic body stump was mobilized in preparation for the pancreaticojejunostomy.
Reconstruction stage
Pancreatic reconstruction was fundamentally performed by end-to-side, duct-to-mucosa anastomosis. First of all, two or three 3–0 coated polyglactin (Vicryl, Ethicon Inc., Somerville, NJ, USA) pancreas-penetrating sutures were placed on the remnant pancreatic parenchyma above the main duct of the pancreas and were not tied with the sutured jejunal seromuscular layer until the anastomosis of the pancreatic duct was complete. Then, for pancreatic duct anastomosis, 3 or 4 interrupted sutures between the corners and posterior walls of the pancreatic duct and the full jejunal layers of the precomposed jejunal hole were placed and tied using 4-0 absorbable, coated, monofilament sutures (Monosyn, B. Braun Surgical S.A., Barcelona, Spain). Then, an internal pancreatic duct stent, fitted to the size of the pancreatic duct, was inserted. The anterior wall of the pancreatic duct was closed in the same way. Two or 3 more pancreas-penetrating sutures were made between the pancreatic parenchyma and the corresponding jejunal seromuscular layer below the main duct of the pancreas. Generally, overall stitches were applied to completely cover the edge of the cut surface of the pancreas with jejunal serosa layers and these stitches hung above and below the main duct of the pancreas until the sutures of the anterior and posterior wall of the pancreatic duct were tied. Then, they were tied all together to fix the remnant pancreatic parenchyma to the jejunal seromuscular layers [
14]. Lastly, the sutured anastomosis was covered with fibrin sealant (Neoveil, Gunze Corp., Kyoto, Japan) (
Fig. 1).
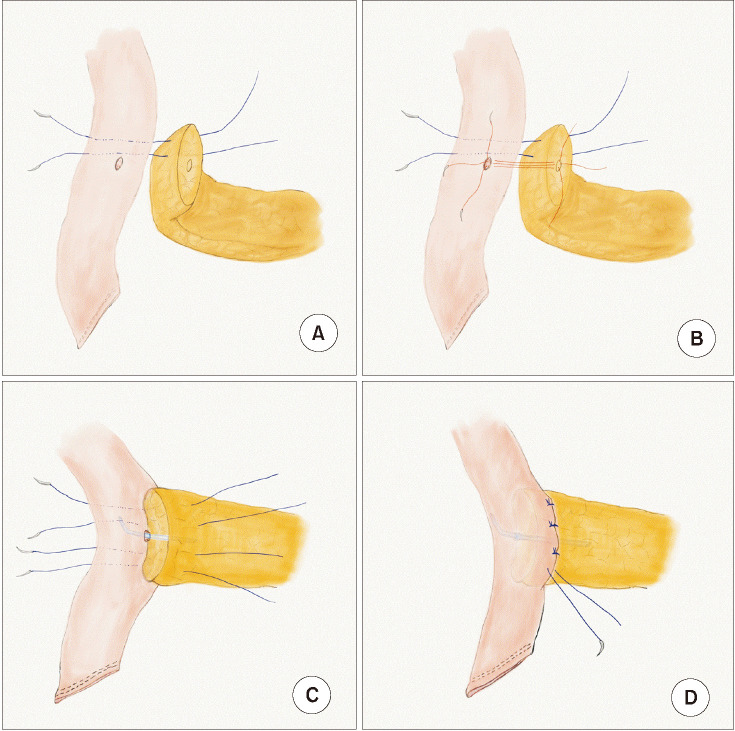 | Fig. 1The pancreatic anastomosis method used. Pancreatic reconstruction was fundamentally performed by end-to-side, duct-to-mucosa anastomosis using several pancreas-penetrating sutures with an internal stent. (A) Four interrupted sutures (between trans-pancreas and sero-muscular layer of a jejunal wall). (B) Duct to mucosa suture. (C) Internal stent. (D) Tying the interrupted sutures.
|
The next reconstruction was an end-to-side hepaticojejunostomy, which was performed with continuous, absorbable, barbed sutures (V-Loc, Medtronic, Minneapolis, MN, USA) to minimize the risk of bile leakage in the posterior wall and interrupted sutures in the anterior wall of the anastomosis. After the completion of the posterior wall anastomosis, internal Silastic stenting according to the size of the hepatic duct was placed over the hepaticojejunostomy. The anterior wall in the hepaticojejunostomy was closed by interrupted 4-0 coated polyglactin (Vicryl) sutures.
Next, a mini-laparotomy was performed on the umbilical port site to extract the specimen, and end-to-side duodenojejunostomy was done in the extracorporeal space through this incision. Two peritoneal drains were placed near the pancreaticojejunostomy and hepaticojejunostomy at the end of the procedure.
In RLPD, after the completion of the laparoscopic resection process, a da Vinci Xi Surgical System (Intuitive Surgical Inc., Sunnyvale, CA, USA) was docked on the right side of the patient, and the assistant surgeon was positioned between the patient's legs. Among the pre-used trocars, only 3 were replaced by a robot, including a trocar for the scope in the umbilicus and two 8-mm trocars at the right hypogastrium and subcostal location, without additional trocar insertion. Robotic reconstruction was performed by docking the robot's arms at these 3 trocar sites (
Fig. 2). The reconstructions were performed in RLPD in the same order and fashion as those performed in the TLPD described above.
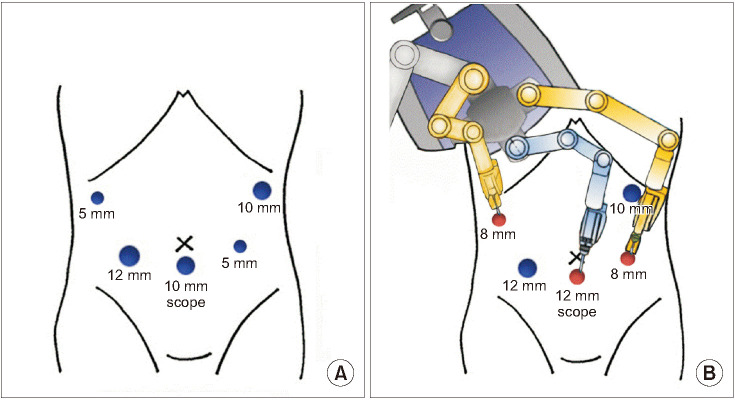 | Fig. 2Docking of the surgical robotic system. Among the pre-used trocars for laparoscopic instruments during the resection stage (A), only 3 were replaced with the robot, including a trocar for the scope in the umbilicus and two 8-mm trocars at the right hypogastrium and subcostal location (B), without additional trocar insertion. Robotic reconstruction was performed by docking the robot arms at these 3 trocar sites.
|
Statistical analysis
The continuous variables are presented either as the mean ± standard deviation or as the median and interquartile range (IQR) as appropriate. The Student t-test was applied to compare the normally distributed variables, whereas the Mann-Whitney U-test was used for nonnormally distributed variables. The categorical data were analyzed using the chi-squared test or Fisher exact test. All statistical analyses were performed using IBM SPSS Statistics ver. 22.0 (IBM Corp., Armonk, NY, USA). A P-value of <0.05 was considered statistically significant.
Go to :

DISCUSSION
Several current studies have discussed the role of robotics in pancreatic surgery, but there have been few studies comparing the perioperative and surgical outcomes of TLPD and RLPD. Our study showed that RLPD significantly reduced the reconstruction stage time. However, there were no significant differences in the POPF, other postoperative morbidities, or mortality. These results showed that robotic reconstruction in MIPD was as safe as conventional laparoscopic reconstruction and could significantly reduce the operation time. This allowed pancreatic surgeons to perform the pancreaticoduodenectomy more easily.
Pancreaticoduodenectomy, one of the most complex and difficult abdominal operations [
15], can be divided into 2 parts; the resection stage and the reconstruction stage. Generally, laparoscopic surgery has limitations such as 2-dimensional imaging, poor surgeon ergonomics, a restricted range of movement [
16], and coarse work without tremor-calibration. In particular, it has been difficult to apply laparoscopic instruments to pancreaticojejunostomy and hepaticojejunostomy because of these drawbacks. The application of a robotic surgical system was believed to provide surgeons with superior, magnified, high-resolution three-dimensional visualization; enhanced dexterity; greater precision; and greater ergonomic comfort [
17]. It enables surgeons to control surgical instruments with accuracy, flexibility, and a wide range of movement [
18]. These robotic surgery properties could make better use of its strengths when reconstruction like pancreaticojejunostomy or hepaticojejunostomy was being performed.
These advantages provided by surgical robots are absolutely necessary, especially when the main duct of the pancreas is very small because it is almost impossible to perform a duct-to-mucosa pancreas anastomosis with just a rigid laparoscopic needle holder and under the low magnification, 2-dimensional, laparoscopic view without the help of tremor-calibration. Using the robot, we could easily find the opening of the main duct as well as perform the fine anastomosis procedure. Also, in the case of bilioenteric anastomosis, if several openings of the hepatic duct are encountered when the proximal hepatic duct is resected to secure a cancer-free margin, anastomosis is not possible with simple laparoscopic tools. But, with the help of the robot, a complex anastomosis could be successfully performed without conversion to open surgery (
Fig. 3).
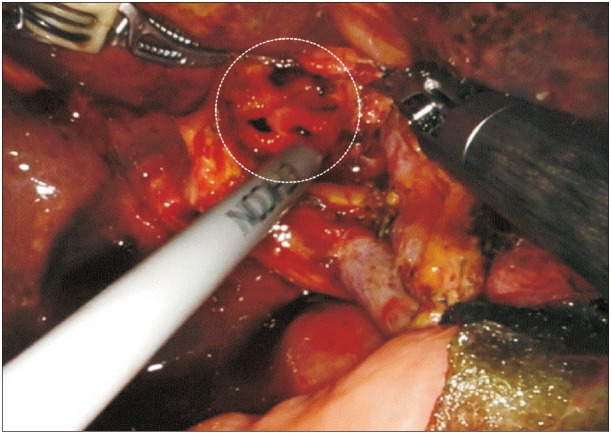 | Fig. 3The multiple hepatic ducts encountered during the bilioenteric anastomosis. In the case of bilioenteric anastomosis, if several openings of the hepatic duct (white circle) are encountered when the proximal hepatic duct was resected to secure a cancer-free margin, anastomosis is not possible with simple laparoscopic tools. However, with the help of the robot, complex anastomosis could be successfully performed without conversion to open surgery.
|
However, compared to laparoscopic instruments, robotic surgical systems have some disadvantages, including a limited range of motion, slow movement, limited available instruments, no tactile sense, difficult control, and difficulty in deciding on an open conversion. These shortcomings could be very disadvantageous for the resection stage of pancreaticoduodenectomy, which involves the entire 4 quadrants of the abdominal space and the deep retroperitoneal space, handling tissues with high bleeding tendency, and controlling a large amount of body fluid during the dissection. Therefore, we applied a hybrid method that combined laparoscopic surgery in the resection stage with robotic surgery in the anastomosis stage. This hybrid method could be an attractive alternative to overcome the disadvantages of both laparoscopic and robotic surgeries. Kim et al. [
19] reported that hybrid pancreaticoduodenectomy had the advantage of reducing the AT and enabling rapid overcoming the learning curve of pancreaticojejunostomy and hepaticojejunostomy.
There were several efficient features of the hybrid method we used. First, since the same trocars used in laparoscopic surgery could be used when switching to robotic surgery in the reconstruction stage, it was unnecessary to insert additional trocars. In particular, since the position of the trocars for the robotic arms was the same as that for the laparoscopy, it provided a familiar surgical environment to the surgeons, making it easier for a novice surgeon to acquire advanced minimally invasive skills, and reducing the use of additional trocars, which was more economical. Secondly, when MIPD was performed, the decision to convert to open surgery occurred mostly in the resection stage. It could be considered that the cost was low when converting to open surgery because the robot was not used from the beginning to this point. Thirdly, a minimum of only 2 instruments was used after docking the robot, a Maryland bipolar forceps that could hold tissue precisely, and a Mega SutureCut needle driver (Intuitive Surgical Inc.) that could do needling and cutting all at once.
A previous study comparing robotic pancreaticoduodenectomy with LPD demonstrated that robotic pancreaticoduodenectomy could reduce the rate of conversion to open procedures, and there were no significant differences in oncologic and postoperative complications [
20]. The study reported the postoperative outcomes of laparoscopic surgery and robotic surgery, especially regarding the surgical results related to the resection stage of the pancreaticoduodenectomy. In this study, we focused on the results of each anastomosis using laparoscopy or a robot.
This study had several limitations. First, the retrospective study design was subject to inherent selection bias. However, all the operations were performed by the same surgical team. All patients were required to be eligible for both the surgical approaches and there were no significant differences in the patient basic characteristics between the 2 groups. Because robotic surgery is generally expensive, the patients chose between the 2 modalities in a relatively unbiased fashion, depending upon their economic situation. In general, TLPD and RLPD were performed sequentially 1:1 in our institution, and more than 20 surgeries were performed each year. All these could reduce the performance bias of the operating surgeons. Second, at the start of the study, the TLPDs were performed after significant experience had already been obtained, whereas there was a learning curve involved with robotic technology. In consideration of the inevitable learning curve for RLPD, we anticipated that the robotic technique would improve the perioperative data of patients when compared to the laparoscopic approach in a further study. Third, this study had a relatively small sample size. However, to the best of our knowledge, this was the first study to investigate the surgical outcomes of RLPD, especially during pancreaticojejunostomy and hepaticojejunostomy assisted by a robot. Though this study provided the perioperative and surgical outcomes of RLPD, further studies on the mid- and long-term outcomes are warranted.
This study analyzed our preliminary experience performing RLPD and showed that the procedure was safe and feasible. The surgical outcome parameters of RLPD, such as the whole operative time and AT, improved, and the incidence of wound infections tended to decrease throughout the experience. The rigorous monitoring of surgical outcomes of anastomosis using robot assistance is fundamental for ensuring patient safety during the adoption of any new surgical technology, especially when a challenging surgical operation such as pancreaticoduodenectomy is performed. A prospective large-scale study with long-term results should be conducted to confirm the results of this study.
Go to :








 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download