Abstract
Purpose
Accurate restaging of the axilla after neoadjuvant chemotherapy (NAC) is an important issue to ensure deescalating axillary surgery in patients with initial metastatic nodes. We aimed to present our results of targeted axillary biopsy (TAB) combined with sentinel lymph node biopsy (SLNB) for axillary restaging after NAC.
Methods
In 64 breast cancer patients who underwent NAC, biopsy-proven positive nodes were marked with clips before NAC, and ultrasound-guided wire localization of clip-marked nodes was performed after NAC. Patients underwent TAB and SLNB for post-NAC axilla restaging.
Results
Identification rates of post-NAC TAB and SLNB were 98.4% and 87.5%, respectively (P = 0.033). Histopathology revealed a nodal pathologic complete response (pCR) rate of 47% in which axillary lymph node dissection (ALND) was avoided. TAB alone and SLNB alone detected residual disease in 29 (85.3%) and 20 (58.8%) patients (P = 0.029), respectively. Whereas rates of up to 97% had been achieved with a combination of TAB and SLNB. The pCR rates after NAC were 64.3% for human epidermal growth factor receptor 2 positive and triple-negative tumors and 13.6% in luminal tumors (P = 0.0002).
Conclusion
Pathologic analysis following TAB combined with SLNB revealed the pCR rates to NAC in a considerable number of patients that provided de-escalation of axillary surgery. A combination of SLNB and TAB was found to be an accurate procedure in establishing residual nodal disease. This combined procedure in patients with initially positive nodes was a reliable method for post-NAC axillary restaging.
Go to : 
The axilla is evaluated by many techniques in patients with previous surgical treatment of breast cancer; such as sentinel lymph node biopsy (SLNB), preoperative ultrasound (US), and US-guided tissue diagnosis. On the other hand, breast surgery is delayed after neoadjuvant chemotherapy (NAC), which is an important treatment phase, especially for patients with node-positive and advanced breast cancer. In patients undergoing NAC, malignant cells can be cleared from initial metastatic nodes in a significant number of patients. Therefore, the evaluation of nodal pathologic response to chemotherapy is a key factor for post-NAC axillary surgery. Previous studies on NAC have shown that rates of pathologic complete response (pCR) of axillary lymph nodes was more than 30% depending on molecular subtypes of the tumor [12345]. Axillary restaging after NAC requires a specific approach with new techniques. The authors reported that US alone could not adequately predict nodal response after NAC. In light of clinical trials, guidelines recommend special techniques for the proper restaging axilla after NAC [36].
Clinical and radiological evidence of good nodal response to NAC resulted in the de-escalation of axillary surgery after chemotherapy. An important issue here is to accurately identify initially positive nodes for restaging the axilla after NAC. Such identification is challenging if nodes are not properly determined and marked before chemotherapy. After NAC, the false-negative rate (FNR) of SLNB alone is high in breast cancer patients with nodal metastasis [78]. Marking clip, radioactive seeds, tattooing with activated charcoal, and wire placement have been used to localize cytologically proven metastatic node [45910111213]. Targeted lymph node biopsy after NAC is strongly dependent on adequately marking initially positive nodes under US guidance.
We hypothesized that targeted axillary biopsy (TAB) with the dual technique is effective for post-NAC axillary restaging. Our TAB comprised pre-NAC identifying and marking positive lymph nodes with clips under US guide, and post-NAC identifying clipped nodes and localizing them with a guidewire under US imaging for targeted excision. In this prospective study, we aimed to present our results of TAB combined with SLNB for axillary restaging after NAC.
Go to : 
A prospective study was conducted on 64 patients with breast cancer who underwent NAC between January 2018 and December 2019. According to the TNM classification of American Joint Committee on Cancer 7th or 8th edition, all patients had clinical T1 to 3, N1, and M0 breast cancer. Institutional Review Board approval (No. TUEK 771-06) was obtained before commencing the study. Full informed consent of patients was obtained before their management. All patients were first evaluated by a breast cancer multidisciplinary team that decided on the eligibility of patients for NAC as appropriate management of their breast cancer with axillary metastasis.
All patients had bilateral mammograms, US of the breast and axilla. Radiologically suspicious breast lesions and axillary nodes were evaluated with core biopsy and/or fine-needle aspiration biopsy (FNAB). Pathological analysis reported the type of breast malignancy, the molecular status of the tumor (expression of estrogen, progesterone and human epidermal growth factor receptors, estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 [HER2]) and ki-67 proliferation index. Cytological analysis after fine needle aspiration (FNA) from axillary nodes established initial nodal status.
Breast cancer cases with up to 3 involved axillary nodes were eligible for TAB. Before chemotherapy, positive lymph nodes were marked under local anesthesia. A marker clip (UltraClip II Tissue Marker, US ribbon, 17 gage × 10 cm; BARD, Tempe, AZ, USA) was placed with US guidance in biopsy-proven metastatic axillary nodes. Clips were inserted into only 1 positive lymph node (ultrasonographically most suspected and largest pathologic) in all patients. The breast radiologist placed the clip within the hypoechoic cortex of the lymph nodes. In order to properly find the right nodes, the location of clipped lymph nodes and their relations with anatomical structures were recorded by US.
The patients received standard chemotherapy regimens (4 cycles of doxorubicin with cyclophosphamide [DC] every 3 weeks followed by weekly taxane-based chemotherapy for 12 weeks in all patients). Four cycles of trastuzumab were administered every 3 weeks with taxane-based chemotherapy in 21 HER2 positive patients before post-NAC surgery. Axillary nodal tumor response was monitored by physical examination and US so that nodal status was established by same experienced breast-dedicated radiologist. Axillary nodal status was monitored after 4 cycles of DC and after taxane-based chemotherapy before post-NAC surgery.
On the day of surgery, clipped nodes were localized, and flexible marking wires (Breast Localization Needles, 20 gage × 10 cm; Geotek, Ankara, Turkey) were placed in the clipped nodes under US by a breast radiologist. Marking wires were used to guide exploration of clipped nodes during axillary surgery, and proper removal of the right nodes.
Five milliliters of a blue dye (isosulfan blue) were injected into the retroareolar breast tissue. Axillary exploration was performed 5 minutes after injection. Blue staining lymph nodes were identified, removed, and sent for pathological examination during surgery
The specimen was first sent for pathological analysis so that frozen section and/or imprint cytology could be performed to detect malignant cells in lymph nodes. If the pathology report was negative for malignancy, no further dissection was performed. If the pathology report revealed metastases in the excised nodes, axillary lymph node dissection (ALND) was performed. Identification rate (IR) of targeted nodes was determined after US and/or radiologic imaging and pathology report. The number of removed nodes was determined and recorded in the specimen by the pathologic examination. The final pathological status of the axilla was established by histopathological analysis. Nodal tumor response to NAC was evaluated in that nodal pCR was defined as the absence of malignant cells in the lymph nodes. Nodal tumor response to NAC was also evaluated according to molecular subtypes. Accuracy of TAB and SLNB combination for restaging the axilla after NAC was assessed with US, surgical exploration, and pathological analysis.
All variables that were expressed as numbers and percentages were compared using Fisher exact test. The P-values of <0.05 were considered statistically significant.
Go to : 
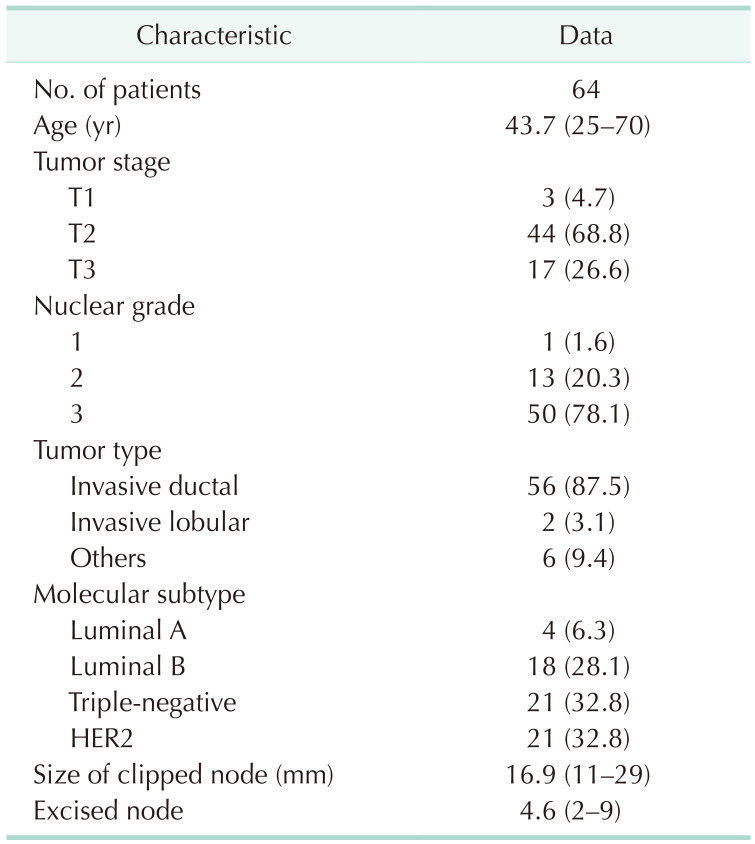
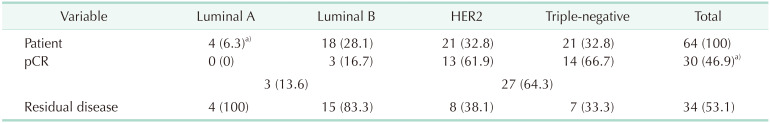
The mean initial size of metastatic nodes was 16.9 mm (range, 11–29 mm). Pathological analysis showed that 87.5% of patients had invasive ductal tumors and 68.7% of patients had T2 tumors. When the molecular subtypes of patients were analyzed, triple-negative (TN) and HER2 tumors accounted for 65.6% of all patients (Table 1).
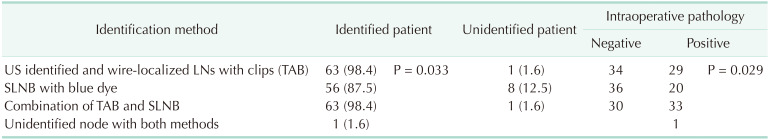
Biopsy-proven initial metastatic nodes marked with clips and localized with a guidewire were identified by US on the day of axillary surgery in 63 patients. Thus, the IR was 98.4% (Table 2).
Sentinel lymph nodes (SLNs) were identified in 56 patients, thus the IR was 87.5%. Clipped nodes were not identified in 1, and blue-dyed nodes in 8 patients (P = 0.033, Table 2). An average of 4.6 (range, 2–9) nodes were removed as targeted and sentinel nodes.
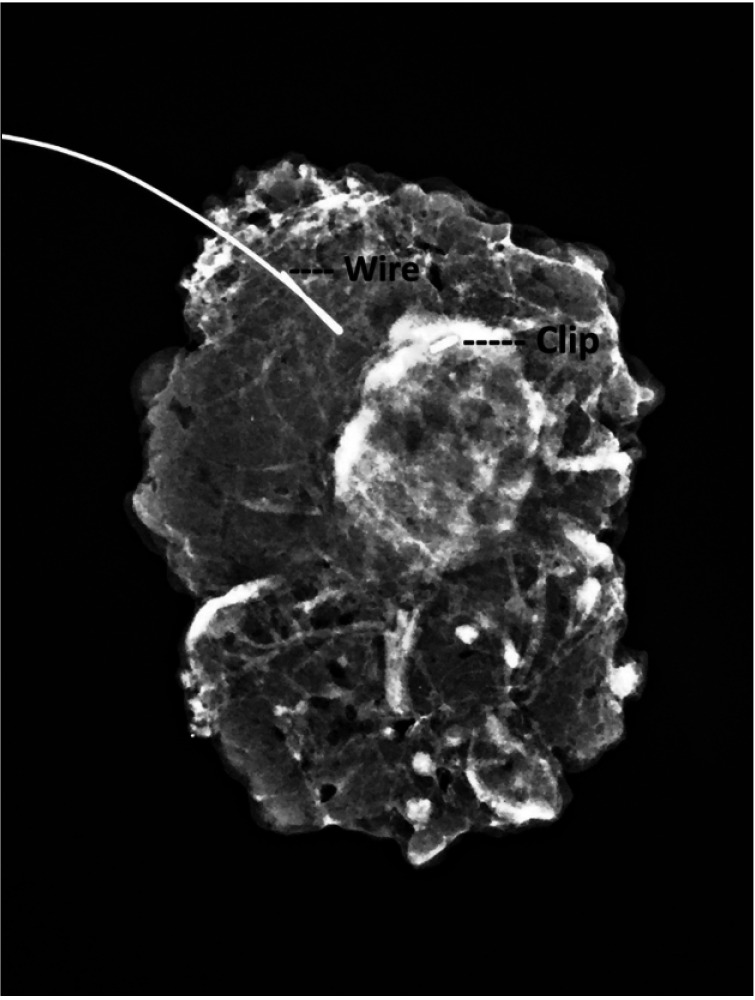
ALND was directly performed in 1 (1.6%) patient with unidentified targeted and sentinel nodes. In 63 patients, marked nodes were excised for cytological analysis during surgery. In all patients, successful clip removal was documented by specimen radiography (Fig. 1).
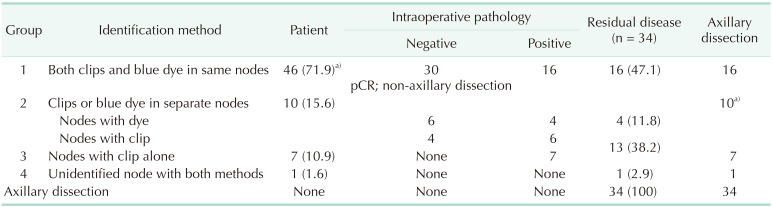
Intraoperative pathological analysis of targeted nodes was negative in 30 patients (46.9%); as such, no further axillary dissection was performed. The final pathology report revealed pCR in these patients (Table 3). The remaining 34 patients had residual disease leading to ALND. Lymph nodes could be identified in 33 of 34 patients. Intraoperative pathology revealed residual disease in nodes with both clip and blue dye of 16 patients (47.1%), and either in clipped nodes alone or in blue dye nodes alone of the remaining 17 patients (Table 3). Wire-localized clipped nodes alone and blue-dyed nodes alone detected residual disease in 29 (85.3%) and 20 (58.8%) of 34 patients (P = 0.029), respectively. Whereas, rates of up to 97% were achieved with the combination of TAB and SLNB. Pathology results according to the identification method of lymph nodes in patients with residual disease could be evaluated in 4 groups (Table 3).
Group 1: This group comprised 46 patients in whom lymph nodes contained both clip and blue dye (coincidence clip and blue dye in the same nodes), of which 16 patients with post-NAC residual disease underwent ALND.
Group 2: This group comprised 10 patients of whom lymph nodes either with clip alone or with blue dye alone were identified separately. Nodes with clip alone were metastatic in 6, and nodes with blue dye alone were metastatic in 4 patients. Thus, all 10 patients underwent ALND.
Group 3: This group comprised 7 patients of whom lymph nodes with clip alone were metastatic. Thus, all 7 patients underwent ALND. In this group, lymph nodes were not identified by SLNB with blue dye.
Group 4: The only patient in whom axillary lymph nodes could not be identified with both methods directly underwent ALND.
According to the molecular subtype classification, 42 patients (65.6%) had TN and HER2 positive tumors. The nodal pCR rate in TN and HER2 tumors was significantly higher than in luminal tumors (P = 0.0002). Sixty-four percent of TN and HER2 positive tumors had nodal pCR (Table 4).
Go to : 
Patients who are candidates for NAC generally have axillary metastasis. In our series, US-guided FNA, and cytological examination successfully verified metastatic nodes before NAC. Axillary status was evaluated by US and US-guided FNAB and/or core biopsy that previous studies had confirmed as successful evaluation of the axilla before NAC [13101114]. Significant changes may occur in the axilla due to pathological response to NAC. In this stage, the most important issue is accurate post-NAC axilla restaging which was previously metastatic. Adequate assessment of nodal pathological response to chemotherapy is crucial to properly determine patients requiring ALND. Breast surgeons could avoid ALND after pathologic evaluation of targeted and sentinel nodes. Which method could accurately restage the axilla after NAC and evaluate the pathological response to chemotherapy?
TAB seems to be an effective method of restaging the axilla after NAC and has emerged as a new staging option in biopsy-proven node-positive patients who convert to node-negative after chemotherapy [3459]. First of all, accurate pre-NAC marking of metastatic nodes is of paramount importance in identifying the correct nodes after NAC. We think that the first step of successful TAB is the effective marking of positive nodes in the pre-NAC period, which, in our study, all such nodes were clip-marked with US guidance. We suggest that the marking clip should be placed within the hypoechoic cortex of the node instead of within the fatty hilum to improve visibility and prevent dislocation. We think that lymph node marking is mandatory before pathologic changes due to chemotherapy in order to detect the correct targets during post-NAC surgery. Morency et al. [15] reported that post-NAC axillary US was not appropriate as a standalone staging procedure in patients who present with N+ breast cancer. Previous studies emphasizing the importance of pre-NAC marking reported that the correct nodes were easily identifiable by US and other methods if metastatic nodes were properly marked before NAC [81112131617]. These procedures should be done by an experienced breast-dedicated radiologist who identifies clipped nodes with a high success rate. Our high IR of clipped nodes confirmed the statement that surgical targets should be clearly designated before chemotherapy for successful TAB. In addition to clip marking before NAC, our dual technique of TAB was completed with wire localization of clipped nodes with US guidance just before surgery, which significantly improved the successful removal of targeted nodes allowing more accurate staging. Thanks to this dual technique, our IR attained 98.4% that TAB was evaluated as an accurate method of restaging the axilla after NAC. Plecha et al. [16] reported that the IR of wire-localized clipped nodes was 97% in 91 patients. If biopsy-proven positive nodes were marked before chemotherapy, IRs of more than 95% would have been reported [38121718].
In addition to TAB, we also used SLNB in combination with targeted excision. Wire localization of pre-NAC clipped nodes was significantly more effective than SLN in the accurate identification of lymph nodes (P = 0.033). Our SLNB-IR of 87.5% showed a significant contribution of SLNB to axillary staging. We can comment that post-NAC combination of both methods provided adequate identification of nodes for accurate restaging of the axilla. In a recent series, IRs of SLN after NAC between 85% and 98% have been reported [101112131920]. Previous studies reported that the combination of TAB with SLN excision increased IR to 100% [34212223]. Simons et al. [24] reported good rates for SLN alone (87.8%), marked node alone (92.8%), and combination procedure (99.3%) when compared with our results of 87.5%, 98.4%, and 98.4%, respectively. Based on high IR values of combined techniques, we can comment that the accuracy of combination procedures was considerably high for post-NAC restaging of the axilla.
Every effort should be spent to enhance the accuracy of TAB and/or SLNB after NAC, and to ensure de-escalating axillary surgery. We can comment that proper evaluation of both clip-marked and blue-dyed nodes was an important predictor of nodal residual disease. ALND remains the standard part of breast surgery for patients who present with node-positive disease. On the other hand, NAC can eradicate axillary metastasis in a significant proportion of patients for which post-NAC restaging of the axilla is crucial in sparing patients the morbidity of ALND. Similarly, in the majority of such series, the eradication rate of nodal metastases was reported between 33% and 45% after NAC in patients with biopsy-proven positive nodes before NAC [12310142526]. Pathological analysis of lymph nodes after surgery revealed nodal pCR to NAC in a considerable proportion (46.9%) of our breast cancer cases. Previous studies using combinations of TAB and SLNB for restaging the axilla in patients with biopsy-proven, node-positive disease reported an axillary pCR rate between 31% and 63%. Therefore, the de-escalating of post-NAC axillary dissection is an important issue in breast cancer surgery [3459202728]. In our series, an average of 4.6 lymph nodes were excised during post-NAC TAB and SLNB. Patients with nodal pCR did not undergo ALND. We know that excising a higher number of SLNs and/or clip-marked nodes in TAB reduces FNR. Previous studies have reported that when more than 2 nodes were excised in TAB and SLNB, FNR was less than 5%, even 2%. After accurate TAB, recurrence rate in the axilla was very low at follow-up [368151729]. Therefore, thanks to TAB, ALND could be avoided in patients with nodal pCR to NAC.
Another aim of targeted axillary surgery is to detect residual disease leading to ALND. Our results showed that a combination of both methods was the most effective modality in detecting residual disease. Targeted clipped nodes alone detected 85.3%, while SLNB alone 58.8% of 34 patients with residual disease. In some patients, 2 methods completed each other for identification of metastatic nodes. Thus, the detection rate of residual disease was up to 97% with combined methods in patients with residual nodal disease. In our study, diagnostic components (of 97%) of metastatic nodes were SLN with blue dye alone, marked node with clip alone, and nodes with both clip and blue dye in 11.8%, 38.2%, and 47.1% of patients with residual nodal disease, respectively. Simons et al. [24] reported the same components as 11%, 23%, and 66%, respectively. We can conclude that a combination of SLNB and TAB was more accurate than either approach alone.
Previous studies reported that several procedures with different materials have been performed to mark positive nodes before NAC [6111213]. In addition to the clip and guidewire, activated charcoal [912], radioactive seeds [5132930], and carbon microparticles [11] were used to mark clipped nodes for post-NAC surgery. Metastatic nodes were marked with clip before NAC, and clipped nodes were marked using 125I seed after NAC [52328]. Complementary methods increased IR of lymph nodes, but not every center is suitable for the application and evaluation of radioactive material. Tattooing with activated charcoal was another method of targeting positive lymph nodes before NAC [912]. Unfortunately, the tattoo does not allow for tracking of targeted nodes by US during the post-NAC period. Whereas clips and guidewire have a clear advantage of being easily visible with US.
The nodal pathological response to chemotherapy was correlated with the tumor molecular subtype. More aggressive types of breast cancer generally give a better response to chemotherapy. According to tumor biology, we found significantly higher nodal pCR rates (64.3%) in patients with TN and HER2 positive disease compared with luminal disease (P = 0.0002). Results of previous studies also confirmed high pCR rates, between 50% and 62%, in patients with HER2 positive and TN tumor compared with luminal subtypes [142425].
Metastatic lymph nodes marked with clip before NAC and wire localization increased IR of correct nodes in TAB after NAC. TAB was found significantly more effective than SLNB with blue dye, but the accuracy of pathologic analysis of such nodes was improved by their combination. Lack of SLNB with the radioisotope method was a partial limitation of our study. A considerable nodal pCR rate to NAC was obtained according to the molecular subtype of the tumor in that a significantly higher pCR rate was found in HER2 and TN tumors. NAC considerably reduced residual tumor burden in axillary lymph nodes. Based on the pathological response to NAC, standard ALND could be avoided in significant proportions of patients despite initial metastatic nodes. The combination of SLNB and TAB was more accurate than either approach alone to establish residual nodal disease. Therefore, this combined procedure in breast cancer patients with initially biopsy-proven positive nodes was a reliable procedure for post-NAC axillary restaging.
Go to : 
References
1. Wong SM, Weiss A, Mittendorf EA, King TA, Golshan M. Surgical management of the axilla in clinically node-positive patients receiving neoadjuvant chemotherapy: a national cancer database analysis. Ann Surg Oncol. 2019; 26:3517–3525. PMID: 31342389.

2. Kim JY, Kim MK, Lee JE, Jung Y, Bae SY, Lee SK, et al. Sentinel lymph node biopsy alone after neoadjuvant chemotherapy in patients with initial cytology-proven axillary node metastasis. J Breast Cancer. 2015; 18:22–28. PMID: 25834607.

3. Simons JM, van Nijnatten TJ, van der Pol CC, Luiten EJ, Koppert LB, Smidt ML. Diagnostic accuracy of different surgical procedures for axillary staging after neoadjuvant systemic therapy in node-positive breast cancer: a systematic review and meta-analysis. Ann Surg. 2019; 269:432–442. PMID: 30312200.
4. Dashevsky BZ, Altman A, Abe H, Jaskowiak N, Bao J, Schacht DV, et al. Lymph node wire localization post-chemotherapy: towards improving the false negative sentinel lymph node biopsy rate in breast cancer patients. Clin Imaging. 2018; 48:69–73. PMID: 29035756.

5. Diego EJ, McAuliffe PF, Soran A, McGuire KP, Johnson RR, Bonaventura M, et al. Axillary staging after neoadjuvant chemotherapy for breast cancer: a pilot study combining sentinel lymph node biopsy with radioactive seed localization of pre-treatment positive axillary lymph nodes. Ann Surg Oncol. 2016; 23:1549–1553. PMID: 26727919.

6. Racz JM, Caudle AS. Sentinel node lymph node surgery after neoadjuvant therapy: principles and techniques. Ann Surg Oncol. 2019; 26:3040–3045. PMID: 31342394.

7. Hartmann S, Reimer T, Gerber B, Stubert J, Stengel B, Stachs A. Wire localization of clip-marked axillary lymph nodes in breast cancer patients treated with primary systemic therapy. Eur J Surg Oncol. 2018; 44:1307–1311. PMID: 29935839.

8. Sutton TL, Johnson N, Garreau JR. Adequate sentinel node harvest is associated with low false negative rate in breast cancer managed with neoadjuvant chemotherapy and targeted axillary dissection. Am J Surg. 2020; 219:851–854. PMID: 32245609.

9. Park S, Koo JS, Kim GM, Sohn J, Kim SI, Cho YU, et al. Feasibility of charcoal tattooing of cytology-proven metastatic axillary lymph node at diagnosis and sentinel lymph node biopsy after neoadjuvant chemotherapy in breast cancer patients. Cancer Res Treat. 2018; 50:801–812. PMID: 28814071.

10. Choi HJ, Kim I, Alsharif E, Park S, Kim JM, Ryu JM, et al. Use of sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with axillary node-positive breast cancer in diagnosis. J Breast Cancer. 2018; 21:433–441. PMID: 30607165.

11. Spautz CC, Schunemann Junior E, Budel LR, Cavalcanti TC, Louveira MH, Junior PG, et al. Marking axillary nodes with 4% carbon microparticle suspension before neoadjuvant chemotherapy improves sentinel node identification rate and axillary staging. J Surg Oncol. 2020; 122:164–169. PMID: 32291774.

12. Kim WH, Kim HJ, Kim SH, Jung JH, Park HY, Lee J, et al. Ultrasound-guided dual-localization for axillary nodes before and after neoadjuvant chemotherapy with clip and activated charcoal in breast cancer patients: a feasibility study. BMC Cancer. 2019; 19:859. PMID: 31470821.

13. Jung SY, Han JH, Park SJ, Lee EG, Kwak J, Kim SH, et al. The sentinel lymph node biopsy using indocyanine green fluorescence plus radioisotope method compared with the radioisotope-only method for breast cancer patients after neoadjuvant chemotherapy: a prospective, randomized, open-label, single-center phase 2 trial. Ann Surg Oncol. 2019; 26:2409–2416. PMID: 31065958.

14. Kim HS, Shin MS, Kim CJ, Yoo SH, Yoo TK, Eom YH, et al. Improved model for predicting axillary response to neoadjuvant chemotherapy in patients with clinically node-positive breast cancer. J Breast Cancer. 2017; 20:378–385. PMID: 29285043.

15. Morency D, Dumitra S, Parvez E, Martel K, Basik M, Robidoux A, et al. Axillary lymph node ultrasound fol lowing neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: results from the SN FNAC Study. Ann Surg Oncol. 2019; 26:4337–4345. PMID: 31605348.
16. Plecha D, Bai S, Patterson H, Thompson C, Shenk R. Improving the accuracy of axillar y lymph node surger y in breast cancer with ultrasound-guided wire localization of biopsy proven metastatic lymph nodes. Ann Surg Oncol. 2015; 22:4241–4246. PMID: 25814365.
17. Cabıoğlu N, Karanlık H, Kangal D, Özkurt E, Öner G, Sezen F, et al. Improved false-negative rates with intraoperative identification of clipped nodes in patients undergoing sentinel lymph node biopsy after neoadjuvant chemotherapy. Ann Surg Oncol. 2018; 25:3030–3036. PMID: 29978371.

18. Laws A, Dillon K, Kelly BN, Kantor O, Hughes KS, Gadd MA, et al. Node-positive patients treated with neoadjuvant chemotherapy can be spared axillary lymph node dissection with wireless non-radioactive localizers. Ann Surg Oncol. 2020; 27:4819–4827. PMID: 32740737.

19. Han A, Moon HG, Kim J, Ahn SK, Park IA, Han W, et al. Reliability of sentinel lymph node biopsy after neoadjuvant chemotherapy in breast cancer patients. J Breast Cancer. 2013; 16:378–385. PMID: 24454459.

20. Kim WH, Kim HJ, Park CS, Lee J, Park HY, Jung JH, et al. Axillary nodal burden assessed with pretreatment breast MRI is associated with failed sentinel lymph node identification after neoadjuvant chemotherapy for breast cancer. Radiology. 2020; 295:275–282. PMID: 32125253.
21. Kim EY, Byon WS, Lee KH, Yun JS, Park YL, Park CH, et al. Feasibility of preoperative axillary lymph node marking with a clip in breast cancer patients before neoadjuvant chemotherapy: a preliminary study. World J Surg. 2018; 42:582–589. PMID: 28808843.

22. Balasubramanian R, Morgan C, Shaari E, Kovacs T, Pinder SE, Hamed H, et al. Wire guided localisation for targeted axillary node dissection is accurate in axillary staging in node positive breast cancer following neoadjuvant chemotherapy. Eur J Surg Oncol. 2020; 46:1028–1033. PMID: 31879050.

23. Kanesalingam K, Sriram N, Heilat G, Ng EE, Meybodi F, Elder E, et al. Targeted axillary dissection after neoadjuvant systemic therapy in patients with nodepositive breast cancer. ANZ J Surg. 2020; 90:332–338. PMID: 31845501.

24. Simons JM, van Pelt ML, Marinelli AW, Straver ME, Zeillemaker AM, Pereira Arias-Bouda LM, et al. Excision of both pretreatment marked positive nodes and sentinel nodes improves axillary staging after neoadjuvant systemic therapy in breast cancer. Br J Surg. 2019; 106:1632–1639. PMID: 31593294.

25. Al-Hattali S, Vinnicombe SJ, Gowdh NM, Evans A, Armstrong S, Adamson D, et al. Breast MRI and tumour biology predict axillary lymph node response to neoadjuvant chemotherapy for breast cancer. Cancer Imaging. 2019; 19:91. PMID: 31878958.

26. Lim GH, Gudi M, Teo SY, Ng RP, Yan Z, Lee YS, et al. Would removal of all ultrasound abnormal metastatic lymph nodes without sentinel lymph node biopsy be accurate in patients with breast cancer with neoadjuvant chemotherapy. Oncologist. 2020; 25:e1621–e1627. PMID: 32537791.

27. Choi HJ, Ryu JM, Kim I, Nam SJ, Kim SW, Yu J, et al. Nomogram for accurate prediction of breast and axillary pathologic response after neoadjuvant chemotherapy in node positive patients with breast cancer. Ann Surg Treat Res. 2019; 96:169–176. PMID: 30941320.

28. Simons JM, Koppert LB, Luiten EJ, van der Pol CC, Samiei S, de Wilt JH, et al. Deescalation of axillary surgery in breast cancer patients treated in the neoadjuvant setting: a Dutch population-based study. Breast Cancer Res Treat. 2020; 180:725–733. PMID: 32180074.

29. Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, Gilcrease MZ, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016; 34:1072–1078. PMID: 26811528.

30. Nguyen TT, Hieken TJ, Glazebrook KN, Boughey JC. Localizing the clipped node in patients with node-positive breast cancer treated with neoadjuvant chemotherapy: early learning experience and chal lenges. Ann Surg Oncol. 2017; 24:3011–3016. PMID: 28766234.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download