Abstract
Purpose
Proximal intestinal bypass (PIB), such as Billroth II or Roux-en-Y gastrojejunostomy after curative distal gastrectomy for gastric cancer induces beneficial effects on glycemic control in patients with type 2 diabetes. We aimed to characterize the long-term evolution of pancreatic beta cells and insulin signaling in target tissue after a PIB procedure.
Methods
Zucker diabetic fatty rats were randomly assigned to the PIB, sham-operated PIB pair-fed, and ad libitum fed groups. Oral glucose tolerance (GT) and plasma insulin levels were measured periodically at 16 weeks postoperatively. Histomorphometric analyses were performed to evaluate changes in islet architectures and intranuclear pancreatic duodenal homeobox 1 (PDX1) expression in beta cells. Insulin signaling changes in visceral adipocytes were measured by the phosphorylated Akt/Akt ratio.
Results
Contrary to the progressively deteriorating GT and plasma insulin levels in sham-operated animals, these were preserved in PIB animals (P < 0.01) at 16 weeks postoperatively. The proportion of the islets having asteroid-like expanding projection was higher in PIB animals than in sham-operated animals (P < 0.01). PIB animals had 3-fold wider fractional area of beta cells (P < 0.01) and 3-fold higher proportion of beta-cell nuclear PDX1 expression (P < 0.01) than sham-operated animals. PIB animals had significantly higher levels of Akt phosphorylation in the visceral adipocytes (P < 0.05). The PIB did not substantially affect weight and food intake postoperatively.
Comorbid diseases have emerged as the leading cause of mortality in gastric cancer patients, especially at the earlier stages [12]. Type 2 diabetes mellitus (T2DM) is one of the most common comorbidities in gastric cancer patients, with a prevalence of over 15% [3]. Patients with T2DM experienced various levels of glycemic control after undergoing curative gastrectomies as treatment for gastric cancers according to the gastrointestinal reconstruction methods [45]. Roux-en-Y gastrojejunostomy more effectively controls hyperglycemia in patients with gastric cancer and T2DM postoperatively, followed by loop gastrojejunostomy, whereas Billroth-I gastroduodenostomy merely affected glycemic control [467]. However, the suggested mechanisms by which loop gastrojejunostomy and Roux-en-Y gastrojejunostomy procedures affect the postoperative glycemic control are not clearly explained.
The metabolic surgical procedures performed to control hyperglycemia in obese patients with T2DM (i.e., Roux-en-Y gastric bypass, biliopancreatic diversion with or without duodenal switch, and one-anastomosis gastric bypass) commonly involve a proximal intestinal bypass (PIB) component [89]. We speculated that the PIB components involving both gastrectomies for gastric cancer and metabolic surgery might share a principal mechanism on glycemic control. Understanding the principal mechanism of improving glycemic control by loop gastrojejunostomy or Roux-en-Y gastrojejunostomy would be crucial to further optimize gastrointestinal reconstruction after gastrectomy for the gastric cancer patient with T2DM.
The development of alterations in glucose homeostasis results from the gradual decline in beta-cell function occurring due to insulin resistance in the liver, muscle, and visceral fat [10]. Zucker diabetic fatty (ZDF; fa/fa) rat expresses severe insulin resistance at a young age and rapidly develops overt diabetic phenotype after a certain compensation period with the increased levels of responsive plasma insulin [1112]. Failure of continuous compensation is mainly caused by an excess of beta-cell death in a setting in which cell replication fails to compensate for the rate of beta-cell loss with aging [1314]. Certain types of antidiabetic pharmaceutical or surgical approaches may result in changes in the islet architecture in the T2DM animal models [1516]. Inadequate knowledge on the PIB-type reconstruction in glucose homeostasis hampers interpretation of its primary role in pancreatic beta cells per se and leaves plenty of room for speculations rather than firm conclusions on its adoption to gastrectomy for the patients with gastric cancer and T2DM.
We hypothesized that the antidiabetic effect of the PIB procedure alone is implicated in primarily improving the pancreatic beta-cell function and simultaneously changing the peripheral insulin signaling. We aimed to characterize how PIB alone affects glucose tolerance (GT), pancreatic beta-cell function, and insulin signaling in the visceral fat in a T2DM rat model.
Ten-week-old male ZDF rats (Charles River, Tokyo, Japan) were individually housed and maintained at 12:12-hour light/dark cycle at constant ambient temperature and humidity (25℃ and 50%–60%, respectively). Rats were fed Purina Lab diet #5008 (WF Fisher & Son, Bound Brook, NJ, USA) for the entire study duration. After 1 week of acclimatization, rats underwent oral glucose tolerance test (OGTT) to examine their diabetes status. Only ZDF rats with an overt diabetic phenotype (2-hour glucose level > 200 mg/dL) were included in the study. The Institutional Animal Care and Use Committee of College of Medicine, Hallym University approved the study protocol (No. HMC2012-1-0706-6).
Twelve-week-old ZDF rats with overt diabetes were randomly assigned to the PIB, sham-operated PIB pair-fed (sham-PF), and sham-operated ad libitum fed (sham-AL) groups. The sham-PF and -AL groups were included to control for caloric intake and weight changes. To investigate the weight-independent mechanism of the surgical interventions, the sham-PF group was included at 1 week after PIB. The 3 groups were followed up at 16 weeks postoperatively. The pancreas was obtained from the subjects of each group at terminal tissue harvest. Additionally, to investigate the natural course of the changes in beta-cell function in ZDF rats, plasma insulin levels were measured in a separate set of unoperated control ZDF rats and normal Sprague-Dawley rats every corresponding time point from ages 6 to 28 weeks.
For the surgical procedure, rats were fasted overnight (12 hours) and anesthetized with 2% isoflurane and oxygen. The PIB procedure was designed to adhere to the concept of the stand-alone stomach-sparing bypass that only excludes the duodenum and proximal jejunum, such as that in human Roux-en-Y reconstruction, which was performed as previously described [17]. Briefly, the duodenum was divided just distal to the pylorus, and the distal segment was closed with number 5-0 Prolene sutures (Ethicon, Piscataway, NJ, USA). The jejunum was divided 5 cm distal to the ligament of Treitz, and the distal limb was anastomosed using number 5-0 Vicryl sutures (Ethicon) to the proximal segment of the duodenum. The continuity of the biliopancreatic limb was restored by end-to-side anastomosis with the alimentary limb 10 cm distal to the gastrojejunostomy site in a Roux-en-Y manner. The abdominal cavity was closed in layers. The laparotomy without experimental bowel manipulation was performed in the shame-operated ZDF rats. The operation time was prolonged to produce a similar degree of anesthetic and surgical stresses as in the respective PIB group.
Subcutaneous injections of buprenorphine (0.01 mg/kg body weight [BW] twice daily for 2 days) were administered to all postoperative rats. Antibiotics were not administered to rats after surgery.
BW and food intake (FI) were measured daily between 9:00 am and 10:00 am throughout the study period. The sham-PF rats were given access to the exact amount of food eaten by the surgical rats on the previous day.
After an overnight fast (12 hours), rats received an oral load of glucose (2 g/kg BW) via oral gavage. Blood samples were collected from the rats' tail vein before (t = 0 minute) and 15, 30, 60, 120, and 180 minutes after glucose administration using Microvette CB 300 K2E tubes (Sarstedt, Nuernbrecht, Germany) containing dipeptidyl peptidase IV inhibitor (Millipore, MA, USA). Blood glucose levels were determined using an automated blood glucose reader (YSI2700 SELECT Biochemistry Analyzer, Biocompare, San Francisco, CA, USA). The tubes were immediately placed on ice, and within 1 hour, the plasma was prepared by centrifugation (2,795 ×g at 4℃) using an Eppendorf bench top centrifuge and stored at −80℃ until assay. GT was calculated as area under the curve (AUC) with t0 starting and t180 ending points for each experiment. Results are expressed as glucose excursion (GE) curves and delta AUC of OGTT. OGTT was performed preoperatively and repeated at 2, 4, 8, and 16 weeks postoperatively.
The bodies of the pancreas were obtained at 16 weeks postoperatively from the subjects of each group at terminal tissue harvest in the first set of studies. The pancreas was removed and fixed by immersion in 10% neutral buffered formalin 24 hours before standard processing and embedding in paraffin wax. The pancreas was embedded to demonstrate the head/tail orientation and maximize the area of tissue sections, which were stained with H&E followed by subsequent histopathological examination. Anti-insulin immunohistochemistry (IHC) was performed to identify beta cells containing cytoplasmic insulin granules using a mouse anti-insulin monoclonal primary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and a biotinylated mouse secondary antibody (Vector Labs, Burlingame, CA, USA) with the strep-ABC/HRP kit (Vector Labs). Insulin immunopositivity was visualized as brown staining of the beta-cell cytoplasm. To evaluate the pancreatic duodenal homeobox 1 (PDX1) activity in the beta-cell nucleus, the sections were immunostained for immunoperoxidase using the ABC kit (Vector Labs) or immunofluorescence. Histochemical demonstration of the infiltration of fibrous tissue in the islets was performed by Masson's trichrome staining.
To evaluate the time-course changes in islet morphology, the pancreas was harvested from ZDF control rats (12- and 20-week-old ZDF rats; n = 6 in each age group). In the H&E-stained sections, the islets were defined as clusters of ≥8 beta cells associated with other morphologically identifiable endocrine cells, and all visualized islets were counted in every section. The proportion of the number of islets showing expanding features (asteroid shape) to the total islets was calculated in each section from the control ZDF rats and every group. Two pathologists counted the total number of islets from each section and the number of islets with expanding features in each section. The proportions of expanding islets in every section generated by each pathologist were averaged (<5% variability in the proportions found between the 2 pathologists). The fractional area of beta cells per unit section was measured. For each pancreas, 2 sections separated by 250 µm were analyzed. Islet images were captured using a MIRAX SCAN (Carl Zeiss, Thornwood, NY, USA) with a 200× objective lens. Light source intensity and image hue, brightness, and saturation were standardized in every section. The beta-cell area was automatically determined using a preset positive pixel count algorithm with MetaMorph software ver. 7.7.4.0 (Molecular Devices, Inc., Sunnyvale, CA, USA). The fractional area of beta cell was expressed by a ratio of the insulin-positive pixel counts to the total pixel counts corresponding to the total pancreatic area in a section. The proportions of the number of anti-insulin positive beta cells per total number of the cells in the islet and the proportion of nuclear PDX1 positive beta cells per total number of the anti-insulin positive beta cells in the islet were also calculated. In the Masson's trichrome-stained sections, the degree of fibrosis of the islet in each group was blindly assessed by 2 pathologists using a semi-quantifying fibrosis scoring system (0, none; 1, mild; 2, moderate; 3, marked; and 4, complete effacement). The fibrosis scores in every section that were estimated by each observer were averaged.
Visceral fat t issues were harvested at 16 weeks postoperatively from ZDF rats in the PIB, sham-PF, and sham-AL groups. Tissues were homogenized in 1 mL of NP40-containing lysis buffer (150-mM NaCl, 50-mM Tris pH 8.0; 5-mM EDTA, 1% NP40) containing protease and phosphatase inhibitors (2-µg/mL leupeptin, 2-µg/mL aprotinin, 1-mM sodium orthovanadate, 10-mM sodium fluoride, and 1-mM phenylmethylsulfonyl fluoride). Tissue extract was cleared by centrifugation (12,000 ×g for 15 minutes). Protein samples were boiled with 4× lithium dodecyl sulfate Nupage sample buffer from Invitrogen (NP0007, Carlsbad, CA, USA) for 2 minutes before loading. Electrophoresis was performed at 80 mA using MES-SDS running buffer from Invitrogen. Transfer was performed at a temperature of 4℃ at 100 V for 90 minutes. Polyvinylidene difluoride membranes were blocked in 5% milk-Tris-buffered saline-T. Anti-pAkt, anti-Akt, and anti-actin antibodies were probed overnight at 4℃. The phosphorylated Akt/Akt ratio of each group was expressed by the percent of the relative intensity of phosphorylated Akt compared to Akt intensity.
Data were analyzed using 1-way analyses of variance (ANOVAs). Significant differences between treatment groups were followed up with post hoc Bonferroni multiple comparison tests. Sequential variations were analyzed by 2-way repeated measures of ANOVA with Tukey post hoc differentiation, where appropriate. Data are expressed as mean ± standard error of the mean. Non-parametric tests (e.g., the Kruskal-Wallis test) were performed if appropriate. A P-value of <0.05 was considered statistically significant. Statistical analyses were performed using Graphpad Prism ver. 9.0 (San Diego, CA, USA).
FI was transiently lower in the PIB group than in the sham-AL group at 2 weeks after surgery (P < 0.05). However, there was no significant difference in average daily FI during the study period between the PIB and sham-AL groups (Fig. 1A). BW in the PIB and sham-PF animals was transiently lower than that of the sham-AL animals at 2 weeks after surgery (P < 0.05). Subsequently, all 3 groups did not show any difference in weight gain at 16 weeks postoperatively (Fig. 1B). The GE curve and delta AUC-OGTT were significantly lower in the PIB group than in sham-operated rats at 2 weeks postoperatively (both P < 0.01) (Fig. 2A, B). PIB animals showed significantly lower delta AUC-OGTT than sham-operated animals at 16 weeks after surgery (P < 0.01) (Fig. 2B). Fasting and oral glucose-challenged plasma insulin levels in the PIB animals were significantly higher than those of the sham-operated animals at 16 weeks postoperatively (P < 0.01) (Fig. 2C, D).
Fasting plasma glucose levels in ZDF rats significantly increased at 8 weeks of age and then after (P < 0.01, Fig. 3A). Plasma insulin levels were significantly increased until 8 weeks of age (5.8 ± 1.7 ng/mL vs. 9.1 ± 3.7 ng/mL, P < 0.01) and significantly decreased until 28 weeks of age (1.0 ± 0.2, P < 0.01) (Fig. 3B). The patterns of plasma insulin decline in the unoperated control ZDF rats were exactly the same as that of the sham-operated animals (Fig 2C, D).
Sixteen weeks after surgery, the islets of the PIB animals aged 28 weeks showed asteroid-like expansions similar to that observed in 12-week-old unoperated control ZDF rats (Fig. 4A, D), whereas the islet of the sham-operated animals and 28-week-old unoperated control ZDF did not show such feature (P = 0.98) (Fig. 4B, C, E). The proportion of expanding islets in the PIB animals was significantly higher than that of the sham-operated animals (P < 0.01) (Fig. 4F).
The areas of asteroid-like expansions of the islets in PIB (Fig. 5A) rats were positively stained by anti-insulin IHC in the matched pancreatic sections (i.e., consistent with the area of islet expansion shown by H&E staining) (Fig. 4A). Fractional area of anti-insulin IHC-positive beta cells per microscopic field unit was significantly higher in PIB rats than in sham-PF and -AL rats (0.029 ± 0.007, 0.008 ± 0.003, and 0.007 ± 0.002, respectively; P < 0.01) (Fig. 5D–G). The proportion of the nuclear PDX1-positive beta cells was significantly higher in PIB animals than in sham-operated animals (0.26 ± 0.03, 0.08 ± 0.04, and 0.09 ± 0.01, respectively; P < 0.01) (Fig. 5H–K). Fibrosis scores in PIB animals tended to be lower than those of the sham-AL animals (1.8 ± 0.4, 1.7 ± 0.5, and 2.4 ± 0.5, respectively; P = 0.07) (Fig. 5L–O).
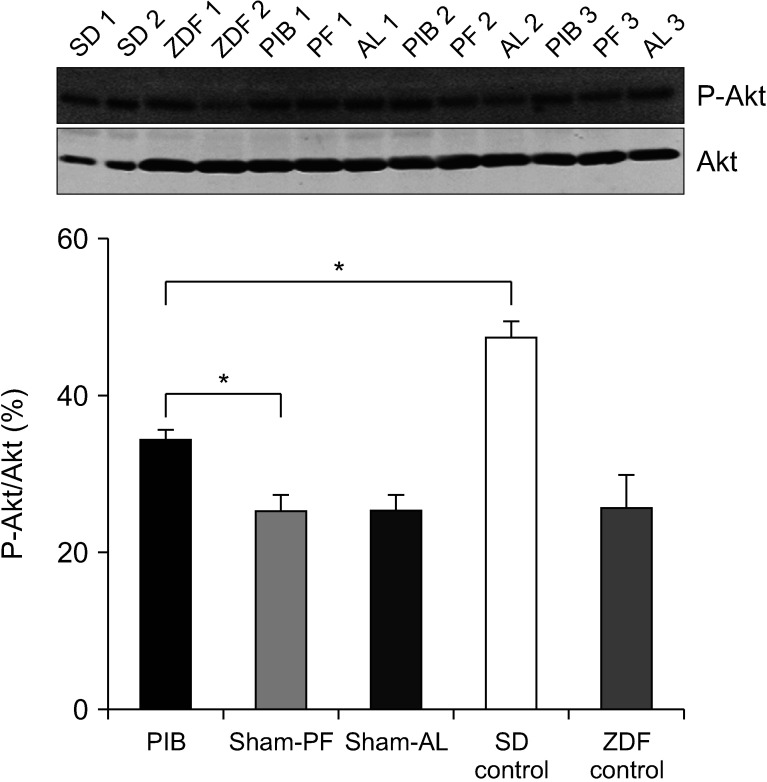
The phosphorylated Akt/Akt ratio was significantly higher in PIB rats than in sham-operated rats (P < 0.05) (Fig. 6).
Previous studies have shown that the patient who underwent gastrectomy for gastric cancer with gastrojejunostomy or Roux-en-Y reconstruction resulting in the exclusion of duodenum and proximal jejunum from food passage enjoyed the antihyperglycemic effect postoperatively [4567]. They emphasized the intrinsic role of bypassed proximal duodenum and jejunum. They often simultaneously claimed the weight loss effect as one of the determinants of glycemic control [6718]. We preserved the pylorus to investigate the sole effect of PIB procedure without any dumping-like symptoms that may reduce the FI and induce a concomitant weight loss in this study. Notably, despite the absence of difference in FI and weight gain profile, the PIB procedure solely ameliorated GT and even successfully maintained better glycemic control over time. We confirmed that the PIB procedure alone could confer GT improvement by a mechanism beyond caloric restriction and weight loss in ZDF rats.
In contrast to the progressively aggravated GT in both the sham-operated groups, which were confirmed in these genetically programmed severe diabetic rats, the PIB procedure preserved distinguishable courses in T2DM progression in the long-term follow-up period. The exclusive role of the stand-alone PIB procedure may be not only a key to treat T2DM but also an interesting area of research in the pathophysiology of diabetes. However, the paucity of data showing long-term consistent outcomes on beta-cell function after duodenal or proximal jejunal bypass surgery leads to making a firm conclusion difficult yet [11920]. The reasons are partly because of the fact that most of the previous studies measured the plasma insulin levels at a single time point postoperatively [1920]. We investigated the time-course variations in plasma insulin concentrations to determine the sustained effect of PIB reconstruction on the pancreatic beta-cell deterioration during the development and progression of T2DM. Interestingly, the PIB procedure eventually preserved the plasma insulin levels, which are primarily responsible for GT throughout the postoperative period. Given the data on greater plasma insulin concentrations coinciding with better GT throughout the study period, we suggested that the long-term effect of the PIB procedure on GT is partly attributable to the preservation of the plasma insulin levels in ZDF rats.
In the PIB animals, over 2/3 of the islets expressed the projections that were 2-fold more frequent compared to the 2 groups of sham-operated animals. The higher plasma insulin levels may be attributable to a higher proportion of these asteroid-like islet expansions after the PIB procedure. The 3-fold wider fractional area of beta cells seemed to be attributable to these striking features of projections of the islets beyond the boundaries and the lesser degenerative changes in islets. In this respect, we focused on the morphological modifications of the islets that might have occurred during the development of diabetes in ZDF rats. Earlier reports demonstrated that duodenojejunal bypass surgery showed hypertrophic islets in 12-week-old ZDF rats [21]. A recent report showed that hypertrophied expanding islets compressed the adjacent exocrine tissues as early as 6 weeks of age in ZDF rats [22]. Farilla et al. [23] reported these budding appearances in a 12-week-old ZDF rat. Notably, incretin (glucagon like peptide-1)-treated 12-week-old ZDF rats presented enhanced irregularity of the margins of the islets and extensive branching out of cells from the periphery of the islets with 3.4-fold higher plasma insulin levels compared to age-matched controls. We also demonstrated that the PIB procedure gave rise to a 3-fold higher PDX1 activity in the beta-cell nucleus. PDX1 plays a crucial role in pancreas development, beta-cell differentiation, and maintaining mature beta-cell function [24]. Interestingly, both groups of the sham-operated animals lost projections of the islets by the same degrees as in the 28-week-old ZDF controls. The mechanism of facilitated beta-cell maturation and prohibited degenerative changes of the islet by the PIB procedure in this genetically programmed progressive T2DM rats needs to be further elucidated in terms of enteroendocrine hormonal milieu, especially the incretin-based mechanisms.
On the other hand, the PIB procedure alone facilitated insulin signaling in visceral adipocytes. Akt, also known as protein kinase B, is a protein kinase that plays a pivotal role in various cellular processes, including cell proliferation, migration, apoptosis, and glucose metabolism [25]. Dysregulation of Akt signaling leads to the development of obesity and T2DM due to defective glucose transport and glycogen synthesis [26]. Considering the induction of Akt activation, we suggest that the PIB procedure can ameliorate glucose homeostasis partly through its substantial role not only in preserving the pancreatic beta-cell viability but also in upregulating the insulin signaling pathway in peripheral tissues [27].
The well-known metabolic surgeries result in reduced gastric volume, followed by gastrointestinal anatomical reconstruction. Likewise, gastrectomy and concomitant small bowel reconstruction also elicit beneficial effects on glucose homeostasis in diabetic gastric cancer patients postoperatively [41828]. In our previous study, postoperative favorable changes in glucose metabolism are greater after loop gastrojejunostomy than after gastroduodenostomy following gastrectomy for gastric cancer [7]. Interestingly, each gastrojejunostomy and conventional Roux-en-Y reconstruction could provide a similar beneficial effect on GT, whereas the gastroduodenostomy did not [4728]. On the other hand, Lee et al. [29] reported that long-limb bypass is essential for improving long-term glycemic control. A multicenter study also demonstrated the favorable glycemic control after the long-limb (>80 cm of each alimentary and biliopancreatic limb) Roux-en-Y bypass reconstruction over conventional loop gastrojejunostomy after gastrectomy despite no difference in body mass index reduction [5]. However, there was a possibility that the long-limb reconstruction might be acting as a 2-faced Janus, showing favorable glycemic control and possible detrimental nutritional deficiency [1], although Park et al. [30] demonstrated no significant differences in nutritional parameters between long-limb and conventional Roux-en-Y reconstructions. Notably, even though we established the Roux-en-Y type PIB procedure consisted of a conventional limb length, which was composed of about 15% of the total small intestine of the rats, we successfully demonstrated the beneficial effect of the PIB on GT and the substantial suggestive mechanism by which it works. In this respect, we carefully propose adopting the salutary effects of PIB reconstruction into the patients with T2DM undergoing gastrectomy for gastric cancer based on the successfully characterized substantial effects of PIB procedure on GT in ZDF rats. Further study is warranted to determine the optimal length of the bypassed limbs.
This study has some limitations. ZDF rats do not completely represent humans with T2DM, because the development of overt diabetes in ZDF rats is genetically programmed, and their disease progression is rapid. That might partly explain why PIB animals failed to maintain the initially improved GT constantly. The ZDF rats are not better surrogates for the non-obese T2DM subjects compared to the Goto-Kakizaki (GK) diabetic rats. However, providing an acceptable range of postoperative survival rate using GK rats, which are much more vulnerable, still remained as an obstacle. In this study, we tested the effects of only Roux-en-Y type reconstruction on beta cells and insulin signaling. The next step, defining the effects of gastrojejunal anastomosis on GT, beta cells, and insulin signaling is warranted to offer an additional option as a reconstruction.
In conclusion, the PIB procedure prevented the further decline in plasma insulin levels in the progressive diabetic animal model, which may be attributable to the preservation of the morphological compensation of the pancreatic islets and beta cells to insulin resistance. This procedure also facilitated insulin signaling in the peripheral target tissues of ZDF rats. The PIB procedure could be considered as a possible reconstruction strategy for diabetic gastric cancer patients.
Notes
Conflict of Interest: Dr. WJ Hyung reports receiving research grants from Medtronic, and is the chief strategic officer and a stockholder of Hutom. Dr. Hyung has also provided consultancy services to Ethicon and Verb Surgical, which are unrelated to the submitted work. The other author declares no conflicts of interest.
References
1. Kunisaki C, Akiyama H, Nomura M, Matsuda G, Otsuka Y, Ono H, et al. Significance of long-term follow-up of early gastric cancer. Ann Surg Oncol. 2006; 13:363–369. PMID: 16485155.

2. Kwon IG, Cho I, Guner A, Kim HI, Noh SH, Hyung WJ. Minimally invasive surgery as a treatment option for gastric cancer in the elderly: comparison with open surgery for patients 80 years and older. Surg Endosc. 2015; 29:2321–2330. PMID: 25480603.
3. Ge Z, Ben Q, Qian J, Wang Y, Li Y. Diabetes mellitus and risk of gastric cancer: a systematic review and meta-analysis of observational studies. Eur J Gastroenterol Hepatol. 2011; 23:1127–1135. PMID: 21934509.
4. Lee W, Ahn SH, Lee JH, Park DJ, Lee HJ, Kim HH, et al. Comparative study of diabetes mellitus resolution according to reconstruction type after gastrectomy in gastric cancer patients with diabetes mellitus. Obes Surg. 2012; 22:1238–1243. PMID: 22179701.

5. Kim JH, Huh YJ, Park S, Park YS, Park DJ, Kwon JW, et al. Multicenter results of long-limb bypass reconstruction after gastrectomy in patients with gastric cancer and type II diabetes. Asian J Surg. 2020; 43:297–303. PMID: 31060769.

6. Kim JW, Cheong JH, Hyung WJ, Choi SH, Noh SH. Outcome after gastrectomy in gastric cancer patients with type 2 diabetes. World J Gastroenterol. 2012; 18:49–54. PMID: 22228970.

7. Guner A, Cho M, Son T, Kim HI, Noh SH, Hyung WJ. Improved glycemic control with proximal intestinal bypass and weight loss following gastrectomy in nonobese diabetic gastric cancer patients. Oncotarget. 2017; 8:104605–104614. PMID: 29262665.

8. Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, et al. Bariatric surgery versus intensive medical therapy for diabetes: 5-year outcomes. N Engl J Med. 2017; 376:641–651. PMID: 28199805.
9. Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Nanni G, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015; 386:964–973. PMID: 26369473.

10. Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care. 2006; 29:1130–1139. PMID: 16644654.
11. Griffen SC, Wang J, German MS. A genetic defect in beta-cell gene expression segregates independently from the fa locus in the ZDF rat. Diabetes. 2001; 50:63–68. PMID: 11147796.
12. Topp BG, Atkinson LL, Finegood DT. Dynamics of insulin sensitivity, -cell function, and -cell mass during the development of diabetes in fa/fa rats. Am J Physiol Endocrinol Metab. 2007; 293:E1730–E1735. PMID: 17895283.

13. Finegood DT, McArthur MD, Kojwang D, Thomas MJ, Topp BG, Leonard T, et al. Beta-cell mass dynamics in Zucker diabetic fatty rats: rosiglitazone prevents the rise in net cell death. Diabetes. 2001; 50:1021–1029. PMID: 11334404.
14. Weir GC, Laybutt DR, Kaneto H, Bonner-Weir S, Sharma A. Beta-cell adaptation and decompensat ion dur ing the progression of diabetes. Diabetes. 2001; 50 Suppl 1:S154–S159. PMID: 11272180.
15. Speck M, Cho YM, Asadi A, Rubino F, Kieffer TJ. Duodenal-jejunal bypass protects GK rats from {beta}-cell loss and aggravation of hyperglycemia and increases enteroendocrine cells coexpressing GIP and GLP-1. Am J Physiol Endocrinol Metab. 2011; 300:E923–E932. PMID: 21304061.
16. Tikellis C, Wookey PJ, Candido R, Andrikopoulos S, Thomas MC, Cooper ME. Improved islet morphology after blockade of the renin- angiotensin system in the ZDF rat. Diabetes. 2004; 53:989–997. PMID: 15047614.

17. Rubino F, Forgione A, Cummings DE, Vix M, Gnuli D, Mingrone G, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006; 244:741–749. PMID: 17060767.

18. Kang KC, Shin SH, Lee YJ, Heo YS. Influence of gastrectomy for stomach cancer on type 2 diabetes mellitus for patients with a body mass index less than 30 kg/m(2). J Korean Surg Soc. 2012; 82:347–355. PMID: 22708096.

19. Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004; 239:1–11. PMID: 14685093.
20. Meirelles K, Ahmed T, Culnan DM, Lynch CJ, Lang CH, Cooney RN. Mechanisms of glucose homeostasis after Roux-en-Y gastric bypass surgery in the obese, insulin-resistant Zucker rat. Ann Surg. 2009; 249:277–285. PMID: 19212182.

21. Pick A, Clark J, Kubstrup C, Levisetti M, Pugh W, Bonner-Weir S, et al. Role of apoptosis in failure of beta-cell mass compensation for insulin resistance and beta-cell defects in the male Zucker diabetic fatty rat. Diabetes. 1998; 47:358–364. PMID: 9519740.

22. Jones HB, Nugent D, Jenkins R. Variation in characteristics of islets of Langerhans in insulin-resistant, diabetic and non-diabetic-rat strains. Int J Exp Pathol. 2010; 91:288–301. PMID: 20384904.

23. Farilla L, Hui H, Bertolotto C, Kang E, Bulotta A, Di Mario U, et al. Glucagon-like peptide-1 promotes islet cell growth and inhibits apoptosis in Zucker diabetic rats. Endocrinology. 2002; 143:4397–4408. PMID: 12399437.

24. Kaneto H, Miyatsuka T, Shiraiwa T, Yamamoto K, Kato K, Fujitani Y, et al. Crucial role of PDX-1 in pancreas development, beta-cell differentiation, and induction of surrogate beta-cells. Curr Med Chem. 2007; 14:1745–1752. PMID: 17627512.
25. Manning BD, Toker A. AKT/PKB signaling: navigating the network. Cell. 2017; 169:381–405. PMID: 28431241.

26. Hers I, Vincent EE, Tavaré JM. Akt signalling in health and disease. Cell Signal. 2011; 23:1515–1527. PMID: 21620960.

27. Beg M, Abdullah N, Thowfeik FS, Altorki NK, McGraw TE. Distinct Akt phosphorylation states are required for insulin regulated Glut4 and Glut1-mediated glucose uptake. Elife. 2017; 6:e26896. PMID: 28589878.

28. Lanzarini E, Csendes A, Lembach H, Molina J, Gutiérrez L, Silva J. Evolution of type 2 diabetes mellitus in non morbid obese gastrectomized patients with Roux en-Y reconstruction: retrospective study. World J Surg. 2010; 34:2098–2102. PMID: 20532768.

29. Lee TH, Lee CM, Park S, Jung DH, Jang YJ, Kim JH, et al. Long-term follow-up for type 2 diabetes mellitus after gastrectomy in non-morbidly obese patients with gastric cancer: the legitimacy of onco-metabolic surgery. J Gastric Cancer. 2017; 17:283–294. PMID: 29302369.

30. Park YS, Park DJ, Kim KH, Park DJ, Lee Y, Park KB, et al. Nutritional safety of oncometabolic surgery for early gastric cancer patients: a prospective single-arm pilot study using a historical control group for comparison. Surg Endosc. 2020; 34:275–283. PMID: 30927123.

Fig. 1
Postoperative changes in food intake (FI) and body weight (BW). The transient difference in FI and BW between PIB and sham-AL rats weakened over time. There were no significant differences in the average daily FI and weight gain between the PIB and sham-operated rats at 16 weeks postoperatively. PIB, proximal intestinal bypass (n = 16); sham-PF, sham-operated paired-fed (n =10); sham-AL, sham-operated ad libitum fed (n = 10). ΔFI = FI (postoperative – preoperative)/preoperative ×100; daily FI = average daily FI during the 16-week study period; BW gain = BW (16 weeks – 1 week before surgery)/1 week before surgery × 100. Data are shown as mean ± standard error of the mean. *P < 0.05 (by analysis of variance test).
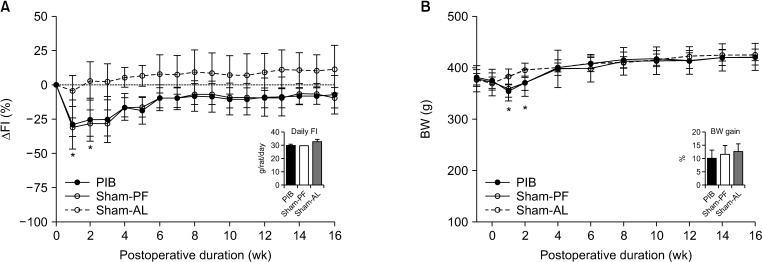
Fig. 2
Effect of the PIB procedure on glucose excursion (GE), glucose tolerance (GT), and the postoperative plasma insulin levels. Only the PIB rats had improvement in GE (A) and GT (B) at 2 weeks postoperatively. The PIB procedure inhibited progressive deterioration of GT, despite the fact that the PIB and sham-PF and -AL animals had similar FI and weight gain. The PIB procedure prevented the gradual decline of fasting (C) and oral glucose-challenged (D) plasma insulin levels of Zucker diabetic fatty rats at 16 weeks postoperatively. PIB, proximal intestinal bypass (n = 16); sham-PF, sham-operated paired-fed (n = 10); sham-AL, sham-operated ad libitum fed (n = 10); AUC-OGTT, area under the curve of oral glucose tolerance test. Δ = (postoperative – preoperative)/preoperative × 100. Data are expressed as mean ± standard error of the mean. *P < 0.01 (by 2-way repeated measures of analysis of variance with Tukey's post hoc test).
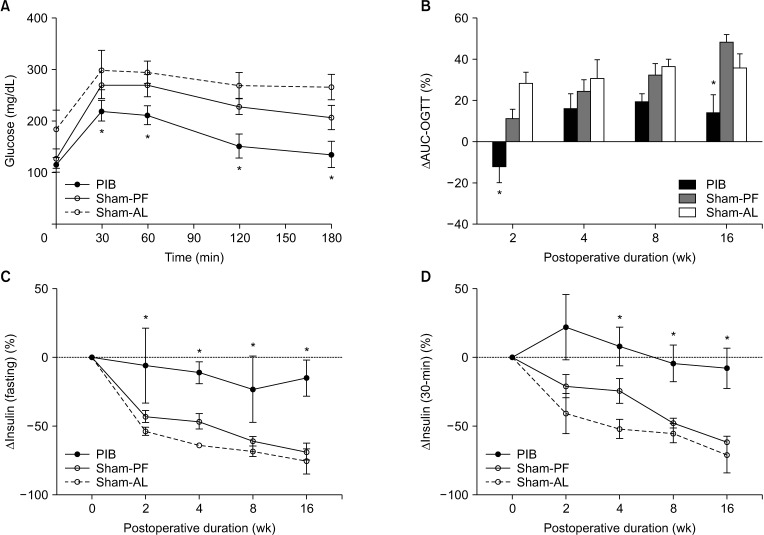
Fig. 3
Characterizing the development of overt diabetes and its progression in Zucker diabetic fatty (ZDF) rats (n = 6). Overt hyperglycemia is accompanied by decreased plasma insulin levels, confirming the decompensation of beta-cell function and progressive nature of insulin deficiency in ZDF rats. SD, Sprague-Dawley rats (n = 6). Data are expressed as mean ± standard error of the mean. *P < 0.01 (by one-way analysis of variance test).
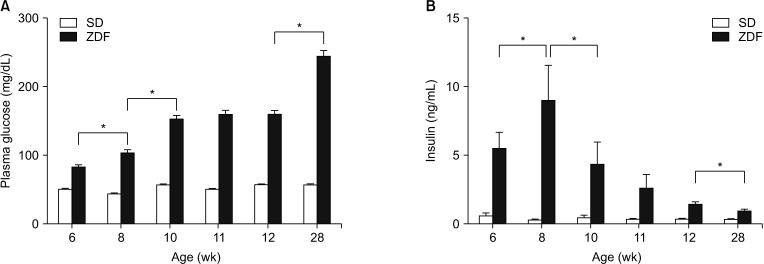
Fig. 4
Effect of the PIB procedure on the preservation of the morphological modification of the islets in Zucker diabetic fatty (ZDF) rats. The majority of islets in the PIB rats aged 28 weeks (A) showed asteroid-like projections (arrow) similar to those seen in 12-week-old control ZDF rats (D) (F). In contrast, these projections of the islets were not frequently seen in the sham-operated animals at 16 weeks postoperatively and 28-week-old control ZDF rats (B, C, E) (F). Expanding islets = number of islets showing asteroid-like expansions/total number of islets in unit pancreas (H&E, ×200). PIB, proximal intestinal bypass (n = 16); sham-PF, sham-operated paired-fed (n = 10); sham-AL, sham-operated ad libitum fed (n = 10). *P < 0.01 (by analysis of variance test).
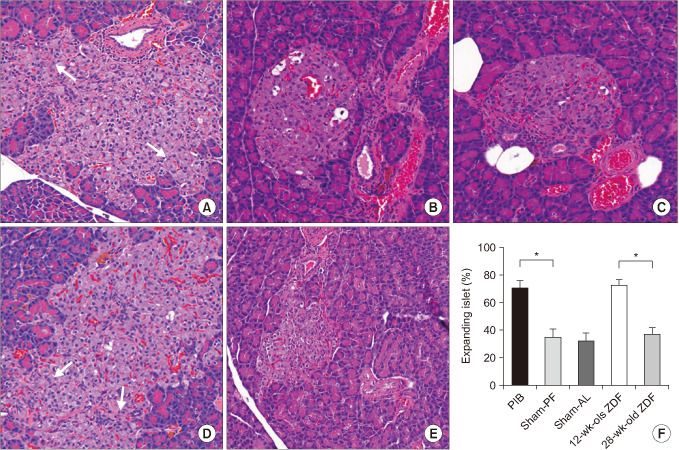
Fig. 5
Changes in the pancreatic beta-cell area, the beta-cell maturation, and the fibrous tissue infiltration in the islets after the PIB procedure in Zucker diabetic fatty rats. In the PIB rats (A), anti-insulin immunohistochemistry (IHC)-positive cells were present beyond the boundaries of typical discrete islets in the majority of islets, which was not observed in the sham-PF (B) and sham-AL (C) rats (anti-insulin IHC staining, pancreas, ×200). The PIB rats (D) had preservation of a wider fractional area of the anti-insulin IHC-positive beta-cell area (fluorescence in green) in the pancreatic islets than the sham-PF (E) and sham-AL (F) rats (G) (anti-insulin IHC staining, pancreas, ×200). The PIB rats (H) had higher anti-pancreatic duodenal homeobox 1 (PDX1) IHC-positivity rate (fluorescence in white) in the beta-cell (in red) of the nucleus (in blue) than the sham-PF (I) and sham-AL (J) rats (K) (anti-intranuclear PDX1 IHC stain, pancreas, ×200). The PIB rats (L) showed a similar marginal area of fibrosis infiltration to the sham-PF (M), but that was lower in the PIB rats than in the sham-AL (N) rats (O) (P = 0.07) (Masson's trichrome staining, pancreas, ×200). PIB, proximal intestinal bypass (n = 16); sham-PF, sham-operated paired-fed (n = 10); sham-AL, sham-operated ad libitum fed (n = 10). *P < 0.01 (by analysis of variance test).
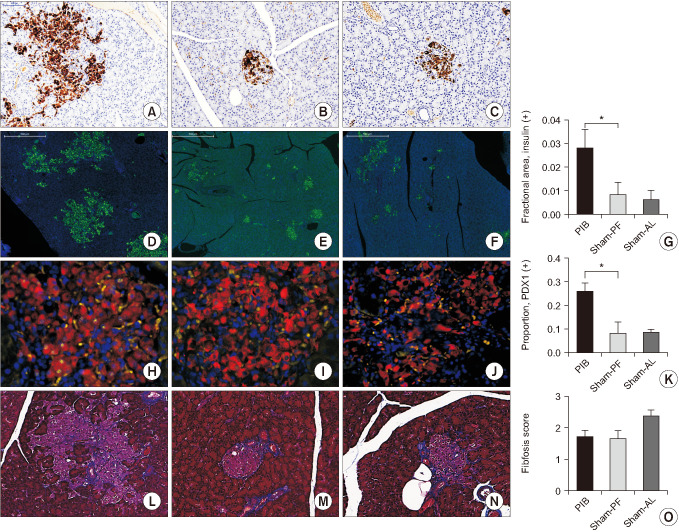
Fig. 6
The effect of the proximal intestinal bypass (PIB) procedure on Akt phosphorylation in visceral fat tissues in Zucker diabetic fatty (ZDF) and Sprague-Dawley (SD) rats. The PIB animals (n = 16) showed an enhanced insulin signaling in the visceral adipocyte than the sham-operated paired-fed (PF, n = 10) and sham-operated ad libitum fed rats (AL, n = 10). Akt phosphorylation in visceral fat was evaluated by Western blotting. Western blots were probed with anti-P-Akt and anti-Akt antibodies. The results are expressed as the percentage of the P-Akt/Akt ratio. Data are expressed as means ± standard error of the mean. *P < 0.01 (by analysis of variance test). P-Akt, phosphorylated Akt.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download