Abstract
Purpose
Colonoscopy is an effective method of screening for colorectal cancer (CRC), and it can prevent CRC by detection and removal of precancerous lesions. The most important considerations when performing colonoscopy screening are the safety and satisfaction of the patient and the diagnostic accuracy. Accordingly, the Korean Society of Coloproctology (KSCP) herein proposes an optimal level of standard performance to be used in endoscopy units and by individual colonoscopists for screening colonoscopy. These guidelines establish specific criteria for assessment of safety and quality in screening colonoscopy.
Methods
The Colonoscopy Committee of the KSCP commissioned this Position Statement. Expert gastrointestinal surgeons representing the KSCP reviewed the published evidence to identify acceptable quality indicators and indicators that lacked sufficient evidence.
Results
The KSCP recommends an optimal standard list for quality control of screening colonoscopy in the following 6 categories: training and competency of the colonoscopist, procedural quality, facilities and equipment, performance indicators and auditable outcomes, disinfection of equipment, and sedation and recovery of the patient.
Colorectal cancer (CRC) is a major cause of morbidity and mortality in Western countries [1] and is the third most common cancer in the Republic of Korea [2]. Although screening can decrease incidence and mortality, CRC screening is only offered to a small portion of the target population worldwide [3]. In 2004, a national cancer screening program for CRC was implemented in Korea. Through this program, all individuals over the age of 50 years can receive an annual fecal occult blood test (FOBT). Any individual who has a positive FOBT result is then offered a colonoscopy examination.
Colonoscopy is a highly effective screening procedure for CRC [4] and can help prevent CRC by detection and removal of precancerous lesions, such as adenomas. Colonoscopy reduces the incidence of CRC, and also reduces mortality by approximately 31%–92%, depending on the population [56789]. The FOBT is inexpensive, widely used for mass screening, and has a high sensitivity in patients with advanced-stage CRC; however, it has a reported sensitivity of only 38.5% in individuals with those stage-T1 CRCs [10]. Although primary screening by colonoscopy is considered a better approach, several issues must be addressed. In particular, when properly performed, colonoscopy is generally safe and accurate, but patients can experience complications or develop interval cancers (cancer during the interval between screening procedures).
The rate of interval CRCs was reported as between 1 per 130 and 1 per 1,000 colonoscopies, thus accounting for 2%–8% of all CRCs [111213141516]. Previous studies reported the rate of interval CRCs in Korea was 7.8%, with an increase over time [17]. The factors responsible for the increasing rate of interval CRC are incomplete resection, failed detection, and aggressive biology of the polyp [141518]. Meticulous inspection of adequately cleaned mucosa and complete removal of preneoplastic lesions are necessary to lower the risk of interval CRC.
In addition, from 2001 to 2005, postcolonoscopy bleeding declined from 6.4 to 2.0 per 1,000 colonoscopies, although the perforation and mortality rates remained stable. A recent meta-analysis reported low overall postcolonoscopy prevalence for perforation (0.5/1,000; 95% confidence interval [CI], 0.4–0.7), bleeding (2.6/1,000; 95% CI, 1.7–3.7), and mortality (2.9/100,000; 95% CI, 1.1–5.5) [19]. Nonetheless, these complications can be serious consequences of the colonoscopy use of a bowel cleansing agent, or use of sedatives.
The participants of the 2018 National Cancer Screening Survey carefully evaluated our screening resources and population screening using colonoscopy to assess the impact on national colonoscopy resources and on complications in patients who are otherwise healthy. According to this survey, which was conducted by the National Cancer Center, about 77% of individuals preferred screening colonoscopies. This suggests that colonoscopy meets one of the requirements of a screening tool, namely that people are willing to use it. However, the most crucial characteristic of a screening colonoscopy program is maintenance of quality in endoscopy units. It is important to use a systematic approach to implement and monitor standards. Colonoscopy programs and units are responsible for quality assurance (QA) [20], and QA strategies are needed to investigate the possible or potential underperformance. Thus, the Executive Board of the Korean Society of Coloproctology (KSCP) commissioned the present Position Statement regarding the certification of accredited endoscopy units for the performance of screening colonoscopies.
Colonoscopy specialists representing the KSCP reviewed the published evidence of different quality indicators and adopted a guideline for each that relies on expert consensus about QA strategies for investigating and monitoring potential underperformance. The panel established evidence-based consensus recommendations based on each QA indicator. Indicators lacking evidence were also determined by expert opinions. The authors discussed the contents at a face-to-face conference and amended the manuscript via e-mail. The recommendation is that the Executive Board of the KSCP monitor quality indicators and use them to license individual colonoscopy and endoscopy units. This Position Statement also proposes references for acceptable colonoscopic practice. The text and tables below describe the details of mandatory items in 6 categories to be evaluated for certification of a qualified endoscopy unit: (1) training and competency of the colonoscopist, (2) facilities and equipment, (3) procedure QA parameters, (4) performance quality and auditable outcomes, (5) infection prevention and adequate reprocessing or disinfection of equipment, and (6) sedation and recovery of the patient. Although not a mandatory item, the recommended item and Korean version of the assessment item for the accredited endoscopic unit have been added to Supplementary Tables 1, 2, 3, 4, 5, 6 and Supplementary Data.
A surgeon who is an endoscopy specialist can be considered one of the most advanced gastroenterology and/or gastrointestinal oncologists. These surgeons can provide optimized treatment, from endoscopy to surgery, to patients with precancerous lesions and to those who need palliative care. To become a surgical endoscopist, the KSCP highly recommends that supervised endoscopy training is administered during the residency or fellowship period so that the physician has integrated knowledge on gastroenterology and/or gastrointestinal oncology (Table 1, Supplementary Table 1).
A colonoscopist who participates in a CRC screening program should be fully trained in colonoscopy, including cecal intubation, biopsy, and polypectomy [2122]. To be considered for credentialing, a colonoscopist must complete a formal 1-year subspecialty training program at a hospital in which 1 certified supervisor is assigned to each trainee. One requirement for achieving competency in endoscopy is the completion of a certain minimum number of colonoscopies. For example, the American Society for Gastrointestinal Endoscopy (ASGE) set the minimum number for assessment of technical competency as 270 supervised colonoscopies [23], and the Korean Society of Gastrointestinal Endoscopy (KSGE) requires 150 procedures at least [24]. Lee et al. [25] found that first-year gastrointestinal fellows reached a cecal intubation rate of 91% after completing 150 colonoscopies and 98% after completing 250 colonoscopies. Another study found that it takes an average of 250 procedures to achieve competence in colonoscopy, based on a cecal intubation rate of 90% and an intubation time of fewer than 15 minutes [26]. On the other hand, the Colonoscopy Academy of the National Cancer Center Korea reported that trainees achieved a 90% cecal intubation rate after performing over 400 colonoscopies [27]. The United Kingdom (UK) Joint Advisory Group on GI Endoscopy replaced its number-based assessment with a combined number and competency assessment. Thus, it requires trainees to perform at least 200 colonoscopies and 200 sigmoidoscopies during their lifetimes, and to achieve a minimum number of procedures that are formally assessed by direct observations of procedural skills [2829]. The Dutch national colorectal cancer screening program (CRCSP) requires a lifetime experience of at least 500 colonoscopies, with at least 200 colonoscopies and 50 polypectomies, as qualification before starting an accreditation program [30]. The KSCP recommends a minimum of 150 successful cecal intubations during 1 year for certification as a colonoscopist. Although it is not a requirement, 100 or more polypectomies over 2 years is recommended for accreditation through the KSCP.
According to the ASGE, mastery in endoscopic procedures depends on the continued practice and performance of adequate numbers of procedures. Performance of fewer than 100 colonoscopies in 1 year is associated with a cecal intubation rate less than 90%. The occurrence of adverse events also depends on a colonoscopist's experience. Three population-based studies demonstrated that the occurrence of colonoscopy-related perforation and bleeding increased significantly in endoscopists who performed fewer than 200–300 colonoscopies per year [3132].
The threshold for maintenance of competence varies from 150 to 300 colonoscopies per year, depending on the guideline. Thus, the National Health Service's Bowel Cancer Screening Program in the UK recommends more than 150 screening colonoscopies in addition to non-screening colonoscopies per year. The Dutch CRCSP recommends that colonoscopists perform CRC screening during at least 200 audited colonoscopies per year [30]. The European guideline for QA in CRC screening recommends that each colonoscopist participating in a CRC screening program perform at least 300 procedures per year to ensure a sufficient sample size for assessment of competence [33]. The KSCP recommends the presence of 1 or more colonoscopists who performed more than 300 colonoscopies during the last 2 years in each endoscopy unit.
The colonoscopists working within a CRC screening program should have good knowledge of the endoscopic characteristics of a healthy large bowel, its anatomy and physiology, and of disease processes. In addition, they must understand the endoscopy instrument, performance of polypectomy or endoscopic mucosal resection, and management of complications after a procedure. Continuing education is also essential so they understand new developments and can improve their performance. The KSCP recommends that colonoscopists complete a minimum of 12 hours of endoscopy-related education, conferences, and lectures every 3 years.
Staff participating in a screening colonoscopy program should also learn necessary new procedures. Thus, all endoscopy units should participate in ongoing quality improvement program that includes action plans covering insufficient performance and acquiring knowledge of new developments in endoscopy.
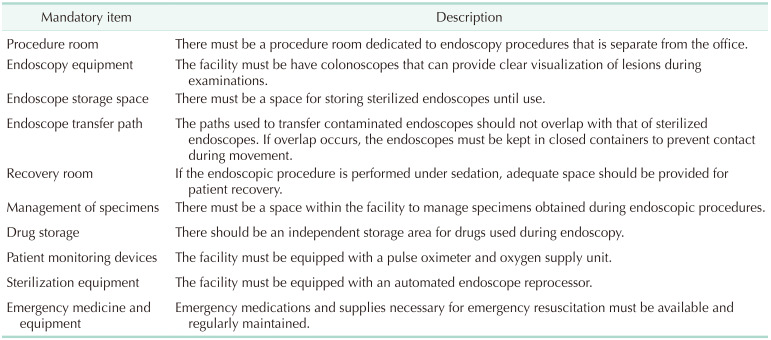
The KSCP considers high-quality and safe endoscopy to depend on adequate maintenance of facilities and equipment and an adequate supply of accessories for a range of specific procedures. However, most existing guidelines do not provide sufficient assessment and accreditation of facilities and equipment. A 1991 guideline for establishing a gastrointestinal endoscopy area briefly referred to the facilities required for endoscopy units [34]. This guideline required a toilet, intravenous equipment, capability for oropharyngeal suction, resuscitation equipment, instrument cabinets, storage area, disinfection of equipment, endoscope and light sources, and electrocautery devices. In 2019, the KSGE published “Accreditation of Qualified Endoscopy Unit,” an update on the guidelines for endoscopy units [35]. This guideline listed 16 mandatory items and 7 recommended items, with each quality indicator classified into 3 categories. The current KSCP guideline lists 10 mandatory items and 13 recommended items for each endoscopy unit. The quality indicators for assessment of an endoscopy unit are classified as endoscopy equipment/accessories, facility/space, and patient monitoring systems (Table 2, Supplementary Table 2).
The large number of endoscopic procedures performed annually indicates the need to perform a QA program of sedation procedures. The monitoring should focus on mitigating patient risk before, during, and immediately after sedation, and should also consider improving procedure outcomes and patient satisfaction (Table 3, Supplementary Table 3).
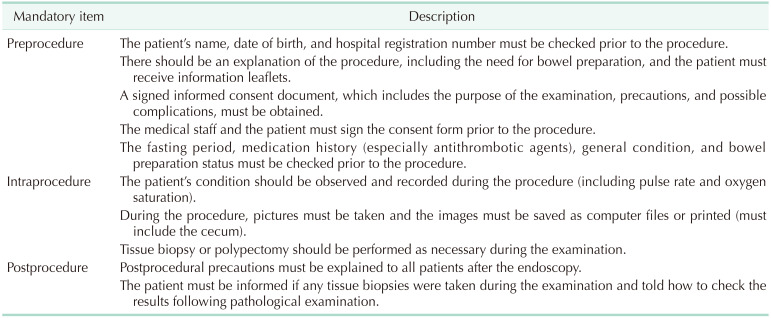
The KSCP recommends a precolonoscopy assessment of the patient that includes receipt of appropriate informed consent, a review of the medical history, a physical examination, and assessment of the risks of sedation and based on preexisting medical conditions. Each patient should be stratified by risk for potential complications to improve management of potential problems related to preexisting medical conditions. An objective method, such as the American Society of Anesthesiologists (ASA) physical status classification, should be used to evaluate the general condition of patients [36]. In addition, the ASGE [37] recommends measuring pre-procedural safety based on how frequently certain issues are addressed, including informed consent, completion and documentation of medical history and physical examination, risk assessment, development of a sedation plan, and performance of a procedural pause or “time-out” [38]. The KSGE recommends patient identification, informed consent, and documentation of preprocedural risk assessment [35]. Use of medications, including antiplatelet agents or anticoagulants, during the fasting period should be monitored. Specifically, the ASA guidelines state that patients should fast a minimum of 2 hours after ingestion of clear liquids and 6 hours after ingestion of light meals before sedation. The discontinuation of an antiplatelet or anticoagulation agent is decided by the colonoscopist based on the risk of bleeding during the procedure [3739].
The intraprocedure time begins with sedation or insertion of the endoscope and ends when the endoscope is removed and the sedation has ended. Following thorough preparation, it is important to monitor the status of a patient during the procedure. The complications from sedatives include phlebitis from intravenous administration, hypoxemia, cardiac arrhythmias, and aspiration. The most common serious problems are cardiopulmonary complications [404142]. The ASGE and ASA guidelines recommend monitoring of pulse rate, blood pressure, and oximetry for early detection of cardiopulmonary complications, such as hypoxemia and respiratory depression [38]. The ASA also recommends use of continuous electrocardiography for patients with significant cardiovascular disease or dysrhythmias. In addition, there should be visual assessment of the patient's ventilatory activity, level of consciousness, and discomfort [43]. The administration and documentation of medications, frequency of using a reversal agent, and rate of early termination because of adverse issues related to sedation are integral quality indicators [44]. Capnography can be used to detect depressed respiratory activity before transient hypoxemia [20]. However, the ASGE concluded there is inadequate data to support the routine use of capnography for patients in moderate sedation [45].
The primary purpose of colonoscopy is the detection and diagnosis of lesions, so complete observation of the colon and abnormalities must be documented. Therefore, images of the cecum and any lesions observed during examination must be saved as a computer file or in a printed version. A tissue biopsy must be performed when there is an abnormal lesion. Storage of 8 photographs of the standard photograph site, including a cecum and tissue biopsy for any lesions, is also recommended as a mandatory part of the procedure by the KSGE [35].
The postprocedural assessment should include following of established discharge criteria, administering proper instructions to the patient, and tracking adverse events. After the sedation has ended and when the discharge criteria are met, the patient should return home. However, before discharge, each patient should be given a description of complications that may occur, from minor symptoms, such as diarrhea and cramping pain, to major symptoms, such as bleeding and perforation [1946]. In addition, the staff should explain the need to contact the hospital if the patient experiences signs or symptoms of delayed adverse events that require immediate medical attention. Patients should also be provided with written discharge instructions that include diet and activity restrictions, an explanation of biopsy results, information about follow-up appointments, and any medication changes.
A quality indicator is an outcome measure supported by sufficient evidence to be strongly recommended as a quality standard. These include cecal intubation rate, withdrawal time, adenoma detection rate, polyp detection rate, quality of bowel preparation, polyp retrieval rate, overall colonoscopy perforation rate, and postpolypectomy bleeding rate. An auditable outcome is a result that should be measured, but is not a quality standard because existing evidence is insufficient. These include sedation level, comfort level, postcolonoscopy CRC, unplanned admission rate, and use of reversal drugs [47]. To be certified, the endoscopy report must describe the information used for auditing the quality indicators. Thus, an endoscopy report must include the date of examination, general characteristics of the patient (name, sex, age, registration number), name of the colonoscopist, drug use, purpose of the examination, bowel preparation state, documentation of cecal intubation and biopsy, predicted diagnosis, and examination time (including insertion time and observation time). When describing a lesion, its location, shape, and size should be reported, and the type of endoscopic procedure and retrieval success should be recorded [48]. Monthly records should be examined to identify the rate of complications, including perforation or bleeding. Use of these reports and documents allows assessment of the major quality indicators (Table 4).
A successful cecal intubation is defined as insertion of the endoscope tip to a point proximal to the ileocecal valve so that the entire cecal pole (including the medial wall) can be visualized and examined. The KSCP recommends that an endoscopist should have a cecal intubation rate of at least 95% during screening colonoscopy of healthy adults. Incomplete procedures, due to poor bowel preparation, obstruction, or severe colitis, and therapeutic colonoscopies should be excluded when determining the cecal intubation rate.
Adequate bowel preparation is necessary for a high-quality colonoscopy, and poor bowel cleansing significantly reduces the detection rate of neoplasia and advanced neoplasia [49]. Moreover, adequate bowel preparation is associated with a greater cecal intubation rate [50]. Adequate bowel preparation may be defined as a score of 6 or more on the Boston bowel preparation scale, 7 or less on the Ottawa bowel preparation scale, or “fair or better” on the Aronchick scale. The ASGE recommends that 90% of patients have adequate bowel preparation and the European Society of Gastrointestinal Endoscopy (ESGE) guideline recommends a minimum of 90% and target of 95% [5152]. In the KSGE guideline, a minimum of 80% adequate bowel preparation is recommended [35]. Given the importance of adequate bowel preparation for screening colonoscopy, the KSCP recommends that adequate bowel preparation rate should be achieved by more than 95% of patients (Supplementary Table 4).
Withdrawal time may be considered a less important quality indicator than adenoma detection rate or cecal intubation rate, but was adopted as a performance indicator because it is associated with the adenoma detection rate [5354]. To achieve an adequate adenoma detection rate, guidelines recommend a mean withdrawal time of 6 minutes or more [55]. Some studies suggest a withdrawal time of 11 minutes or longer to increase the detection rate of proximal serrated polyps [56], but others claim that the net inspection time, excluding cleansing and suction time, should be at least 6 minutes [54]. However, because it is difficult to precisely measure inspection time, it is usually estimated based on a mean withdrawal time of 6 minutes or more when used as a supportive measure for adequate identification of pathology in a patient with negative results. The mean withdrawal time is calculated as the sum of the total withdrawal time divided by the number of colonoscopies performed that do not require a procedure (Supplementary Table 4).
The polyp detection rate, defined as the rate at which 1 or more polyps is detected and removed during colonoscopy, correlates with the adenoma detection rate [5758]. An advantage of recording the polyp detection rate rather than the adenoma detection rate is that it does not require manual entry of pathology data. Thus, many previous studies examined the potential use of the polyp detection rate as a quality indicator because it is measured during the colonoscopy, in contrast to the adenoma detection rate, which requires subsequent analysis. The ASGE recommended adenoma detection rate benchmarks of 25% for males and 15% for females, but did not recommend polyp detection rate as a quality indicator [52]. The ESGE recommended a polyp detection rate of 40% or more as a minor performance measure [59]. Patients who received a colonoscopy from a doctor whose polyp detection rate was more than 30% had a lower incidence of proximal interval cancer than those examined by a doctor whose polyp detection rate was 10% (odds ratio, 0.61; 95% CI, 0.42–0.89) [60]. Another study reported the adenoma detection rate/polyp detection rate ratio was 71.3% and this was unchanged for colonoscopists in different polyp detection rate quartiles [61]. Therefore, the KSCP decided to set a polyp detection rate of 40% or more as a performance quality indicator, and did not use the adenoma detection rate because of the requirement for pathological examination (Supplementary Table 4).
Most of the quality indicators described above assess the accuracy of the examination and monitor the complication rate (bleeding after polypectomy and perforation) because these are essential for assessment of patient safety and acquisition of reliable results. Bleeding is the most common complication of polypectomy, and about 5% of colonoscopic perforations are fatal. Therefore, documentation of adverse events, such as perforation with or without a procedure, bleeding after polypectomy, readmission, and mortality is important. The ESGE guideline suggested a complication rate less than 0.5%, but did not suggest minima for bleeding rate or perforation rate [48]. On the other hand, the ASGE guideline recommended that the bleeding rate after polypectomy should be below 1%, and that the perforation rate should be below 0.2% for all colonoscopies and below 0.1% for screening colonoscopies. The UK guideline recommended that the bleeding rate should be less than 0.5% and the perforation rate after polypectomy should be less than 0.2% [62]. The KSGE guideline did not establish an appropriate endpoint for the complication rate, and it was recommended to describe monthly statistics of patients who received a blood transfusion, hospitalization, or surgery due to a complication [35]. The KSCP recommends a perforation rate below 0.1% and a moderate or severe bleeding rate after polypectomy less than 0.5%. Moderate or severe bleeding is defined as bleeding that necessitates an intervention or operation (Supplementary Table 4).
Several studies reported infections due to inadequate reprocessing of endoscopes and other instruments [6364], although there are no reliable data on the incidence of endoscope-associated infections. It is essential to use high-quality procedures to ensure prevention of infection so that screening colonoscopy procedures are safe. Hence, several consensus guidelines recommend use of a disinfection and reprocessing protocol to prevent infections and use of infection control professionals [3565666768]. The endoscopy room should have written policies and procedures for infection prevention that reflect the current standard guidelines (Table 5).
In the Republic of Korea, 4 organizations certified an “Endoscope cleaning and disinfection guideline,” and the KSCP prepared “Disinfecting guidelines for endoscopy cleaning and disinfection” [69]. According to these written policies, endoscope handling should be performed by qualified practitioners with specific training and experience, and reprocessing should be performed immediately after examination. Immediately after an endoscopy procedure, foreign substances on the outer surface of the endoscope should be wiped off and a detergent solution with suction should be used to clean the inside of the aspiration channel. The insufflation/infusion button, aspiration button, and forceps plug should be detached from the endoscope before cleaning. Then, the interior of the aspiration and forceps channels should be cleaned with a brush.
After manual precleaning of the endoscope, it should be cleaned and disinfected using an automated endoscope reprocessor [30]. The solution used in this process should be a high-quality disinfectant/sterilizing agent capable of destroying all vegetative microorganisms, mycobacteria, small or nonlipid viruses, medium or lipid viruses, fungal spores and some, but not all, bacterial spores. The disinfectant should be routinely tested to ensure the minimum effective concentration of the active ingredient. Strict adherence to the manufacturer's requirements for the reprocessor is critical for the maintenance of appropriate disinfection. Irrigation and drying after disinfection should also be performed in accordance with the instructions.
All of these procedures should be recorded in a “Process Cleaning and Disinfection Management Ledger.” Each unit should have a designated sequence for the safe physical movement of dirty endoscopes and other equipment. The instrument used in the mucosa (e.g., biopsy forceps, polypectomy snares, knives for gastrointestinal treatment, and puncture needles) are in the category of high infection risk. Thus, these instruments must be disposable or sterilized before reuse. For reusable products, sufficient cleaning and sterilization according to the manufacturer's instructions are required. The safety of the colonoscopist and others involved in the procedure must also be protected. Gloves and an impervious gown should be worn by each staff member who is directly engaged in patient care during the procedure.
Sedation during colonoscopy relieves the patient's anxiety, and diminishes the pain, discomfort, and memory of the examination, thereby improving patient tolerance. Despite the benefits of sedation, adverse events such as cardiopulmonary dysfunction can occur depending on patient characteristics and procedural variables. Therefore, the patient should provide informed consent for administration of sedation following a discussion of the benefits and risks (Table 6).
Conscious sedation, in which a patient can respond to verbal or light tactile stimulation while maintaining spontaneous breathing and cardiovascular function, is the goal for endoscopic procedures. Achieving conscious sedation may vary depending on the characteristics of the patient and the doses and types of sedatives. Thus, it is essential to assess a patient's level of consciousness and vital signs before the procedure begins, after administration of sedative-analgesic agents, during initial recovery, and just before discharge. At a minimum, there should be monitoring of heart rate, pulse oximetry, and visual assessment of ventilatory activity. Assessment of blood pressure should be performed before injection of the sedative agent(s) and during the initial recovery. During colonoscopy, electrical assessment of blood pressure is not recommended because it can interfere with the sedation and may reduce patient comfort. The KSGE guideline recommended a pulse oximetry as an essential device for monitoring, and mentioned that vital signs including oxygen saturation should be recorded during a procedure [35]. The ASGE guideline recommends use of minimal patient monitoring so changes in pulse, blood pressure, ventilatory status, cardiac electrical activity, and level of sedation can be detected before any clinically significant events [43]. There is no evidence that capnography during endoscopic procedures with improved the safety of patients during moderate sedation (Table 6) [70].
Even after the colonoscopy is over, there is a remaining risk of complications due to use of the sedative drugs. The effect of sedative agents may persist due to metabolic delay, so the level of consciousness, blood pressure, pulse rate, and oxygen saturation should be monitored during recovery. Discharge criteria should be used to assess the recovery from sedation, and the patient must be conscious and oriented with normal vital signs before leaving the endoscopy unit. Other guidelines use several discharge criteria to evaluate recovery from sedation, such as the modified Aldrete score and the Post-Anesthetic Discharge Scoring System (PADSS) [7172]. A score of 8 or higher on the modified Aldrete score and of 9 or higher on the PADSS indicate the patient can be discharged [727374].
Moderate sedation regimens typically use a benzodiazepine. Most endoscopists favor midazolam because patients experience a rapid onset of action and rapid recovery after withdrawal, and because of its high amnestic properties and the availability of antagonists [75]. Flumazenil is a specific benzodiazepine antagonist that should be readily available in every endoscopy unit (Supplementary Table 6).
Sedation using propofol can provide a faster recovery and discharge, a more rapid sedation and ambulation, and improved patient satisfaction [76]. However, propofol has a narrow therapeutic window, and there is risk for complications if it is not administered appropriately. The main adverse effects are disturbances in cardiopulmonary physiology, such as bradycardia, hypotension, and hypoxemia [77]. Hence, additional training and monitoring may be needed to allow the safe administration of propofol. The KSCP recommends that at least one person in an endoscopy unit has expertise in the use of propofol for sedation (Supplementary Table 6).
The guidelines presented here aim to establish an optimal standard level of performance for endoscopy units and individual colonoscopists who perform screening colonoscopies. Furthermore, this Position Statement provides guidance on measuring clinical outcomes and the area(s) of performance that influence those outcomes. Ultimately, this statement can be refined toward evidence-based best practice. The next step is to implement these key performance measures in endoscopy units throughout the Republic of Korea. We recommend individual colonoscopists, as well as the heads of endoscopy units, start implementation of these performance measures without delay.
As these guidelines for screening colonoscopy are implemented, new data on efficacy and safety will allow updating of these guidelines. In particular, we expect that certain guideline performance domains, such as those regarding the facilities and equipment of endoscopy units, will continue to be upgraded as data becomes available.
ACKNOWLEDGEMENTS
The authors thank the members of the Colonoscopy Committee of the Korean Society of Coloproctology for conception and design, data analysis and interpretation, and final approval of the manuscript.
SL: Department of Surgery, Good-jang Hospital, Seoul, Korea. DKS, KSH: Center for Colorectal Cancer, National Cancer Center, Goyang, Korea. SHM: Department of Surgery, Seoul National, University College of Medicine, Seoul, Korea. BHK: Department of Surgery, St. Vincent's Hospital, The Catholic University, Suwon, Korea. HJS: U and Hang Surgery, Yongin, Korea. SS: Department of Surgery, CHA clinic, Incheon, Korea. SIL: Department of Surgery, Korea University Guro Hospital, Seoul, Korea. DHC: Department of Surgery, Hansarang Hospital, Ansan, Korea.
Notes
Author Contribution:
Conceptualization: WKK, RS, SHM, SIL, SL, DHC, KSH, DKS.
Formal Analysis: WKK, BHK, SHM, HJS, RS, SL, DHC, KSH, DKS.
Investigation: WKK, BHK, SHM, HJS, RS, SL, DHC, KSH, DKS.
Methodology: WKK, BHK, SHM, HJS, RS, SIL, SL, DHC, KSH, DKS, SS.
Project Administration: All authors.
Writing — Original Draft: RS.
Writing — Review & Editing: WKK, SHM, HJS, RS, SIL, SL, SS.
References
1. Stewart BW, Kleihues P. World cancer report 2003. Lyon: IARC Publications;2003.
2. Jung KW, Won YJ, Kong HJ, Lee ES. Prediction of cancer incidence and mortality in Korea, 2019. Cancer Res Treat. 2019; 51:431–437. PMID: 30913864.

3. Schreuders EH, Ruco A, Rabeneck L, Schoen RE, Sung JJ, Young GP, et al. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015; 64:1637–1649. PMID: 26041752.

4. Bretthauer M, Kaminski MF, Løberg M, Zauber AG, Regula J, Kuipers EJ, et al. Population-based colonoscopy screening for colorectal cancer: a randomized clinical trial. JAMA Intern Med. 2016; 176:894–902. PMID: 27214731.
5. Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009; 150:1–8. PMID: 19075198.

6. Kahi CJ, Imperiale TF, Juliar BE, Rex DK. Effect of screening colonoscopy on colorectal cancer incidence and mortality. Clin Gastroenterol Hepatol. 2009; 7:770–775. PMID: 19268269.

7. Manser CN, Bachmann LM, Brunner J, Hunold F, Bauerfeind P, Marbet UA. Colonoscopy screening markedly reduces the occurrence of colon carcinomas and carcinoma-related death: a closed cohort study. Gastrointest Endosc. 2012; 76:110–117. PMID: 22498179.

8. Nishihara R, Wu K, Lochhead P, Morikawa T, Liao X, Qian ZR, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013; 369:1095–1105. PMID: 24047059.

9. Zauber AG, Winawer SJ, O'Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012; 366:687–696. PMID: 22356322.

10. Sohn DK, Jeong SY, Choi HS, Lim SB, Huh JM, Kim DH, et al. Single immunochemical fecal occult blood test for detection of colorectal neoplasia. Cancer Res Treat. 2005; 37:20–23. PMID: 19956505.

11. Burr NE, Derbyshire E, Taylor J, Whalley S, Subramanian V, Finan PJ, et al. Variation in post-colonoscopy colorectal cancer across colonoscopy providers in English National Health Service: population based cohort study. BMJ. 2019; 367:l6090. PMID: 31722875.

12. Cheung KS, Chen L, Seto WK, Leung WK. Epidemiology, characteristics, and survival of post-colonoscopy colorectal cancer in Asia: a population-based study. J Gastroenterol Hepatol. 2019; 34:1545–1553. PMID: 30932240.

13. Forsberg A, Hammar U, Ekbom A, Hultcrantz R. Post-colonoscopy colorectal cancers in Sweden: room for quality improvement. Eur J Gastroenterol Hepatol. 2017; 29:855–860. PMID: 28410353.
14. Murthy SK, Benchimol EI, Tinmouth J, James PD, Ducharme R, Rostom A, et al. Temporal trends in postcolonoscopy colorectal cancer rates in 50- to 74-year-old persons: a population-based study. Gastrointest Endosc. 2018; 87:1324–1334. PMID: 29317271.
15. Pedersen L, Valori R, Bernstein I, Lindorff-Larsen K, Green C, Torp-Pedersen C. Risk of post-colonoscopy colorectal cancer in Denmark: time trends and comparison with Sweden and the English National Health Service. Endoscopy. 2019; 51:733–741. PMID: 31174223.

16. Subramaniam K, Ang PW, Neeman T, Fadia M, Taupin D. Post-colonoscopy colorec t al cancers ident i f ied by probabilistic and deterministic linkage: results in an Australian prospective cohort. BMJ Open. 2019; 9:e026138.
17. Cha JM, Kim HS, Kwak MS, Park S, Park G, Kim JS, et al. Features of postcolonoscopy colorectal cancer and survival times of patients in Korea. Clin Gastroenterol Hepatol. 2019; 17:786–788. PMID: 29966709.

18. Cheung D, Evison F, Patel P, Trudgill N. Factors associated with colorectal cancer occurrence after colonoscopy that did not diagnose colorectal cancer. Gastrointest Endosc. 2016; 84:287–295. PMID: 26827612.

19. Reumkens A, Rondagh EJ, Bakker CM, Winkens B, Masclee AA, Sanduleanu S. Post-colonoscopy complications: a systematic review, time trends, and meta-analysis of population-based studies. Am J Gastroenterol. 2016; 111:1092–1101. PMID: 27296945.

20. Qadeer MA, Vargo JJ, Dumot JA, Lopez R, Trolli PA, Stevens T, et al. Capnographic monitoring of respiratory activity improves safety of sedation for endoscopic cholangiopancreatography and ultrasonography. Gastroenterology. 2009; 136:1568–1576. PMID: 19422079.

21. Atkin W, Rogers P, Cardwell C, Cook C, Cuzick J, Wardle J, et al. Wide variation in adenoma detection rates at screening flexible sigmoidoscopy. Gastroenterology. 2004; 126:1247–1256. PMID: 15131784.

22. Thomas-Gibson S, Bassett P, Suzuki N, Brown GJ, Williams CB, Saunders BP. Intensive training over 5 days improves colonoscopy skills long-term. Endoscopy. 2007; 39:818–824. PMID: 17703392.

24. Moon HS, Choi EK, Seo JH, Moon JS, Song HJ, Kim KO, et al. Education and training guidelines for the Board of the Korean Society of Gastrointestinal Endoscopy. Clin Endosc. 2017; 50:345–356. PMID: 28783925.

25. Lee SH, Chung IK, Kim SJ, Kim JO, Ko BM, Hwangbo Y, et al. An adequate level of training for technical competence in screening and diagnostic colonoscopy: a prospective multicenter evaluation of the learning curve. Gastrointest Endosc. 2008; 67:683–689. PMID: 18279862.

26. Sedlack RE, Coyle WJ. ACE Research Group. Assessment of competency in endoscopy: establishing and validating generalizable competency benchmarks for colonoscopy. Gastrointest Endosc. 2016; 83:516–523. PMID: 26077455.

27. Oh JR, Han KS, Hong CW, Kim BC, Kim B, Park SC, et al. Colonoscopy learning curves for colorectal surgery fellow trainees: experiences with the 15-year colonoscopy training program. Ann Surg Treat Res. 2018; 95:169–174. PMID: 30310799.

28. Ward ST, Mohammed MA, Walt R, Valori R, Ismail T, Dunckley P. An analysis of the learning curve to achieve competency at colonoscopy using the JETS database. Gut. 2014; 63:1746–1754. PMID: 24470280.

29. Jones RP, Stylianides NA, Robertson AG, Yip VS, Chadwick G. National survey on endoscopy training in the UK. Ann R Coll Surg Engl. 2015; 97:386–389. PMID: 26264093.

30. Bronzwaer ME, Depla AC, van Lelyveld N, Spanier BW, Oosterhout YH, van Leerdam ME, et al. Quality assurance of colonoscopy within the Dutch national colorectal cancer screening program. Gastrointest Endosc. 2019; 89:1–13. PMID: 30240879.

31. Singh H, Singh G. Inequities in colonoscopy: variation in performance and outcomes of colonoscopy. Gastrointest Endosc. 2009; 69:1296–1298. PMID: 19481650.

32. Chukmaitov A, Bradley CJ, Dahman B, Siangphoe U, Warren JL, Klabunde CN. Association of polypectomy techniques, endoscopist volume, and facility type with colonoscopy complications. Gastrointest Endosc. 2013; 77:436–446. PMID: 23290773.

33. European Colorectal Cancer Screening Guidelines Working Group. Patnick J, Segnan N, Atkin W, Halloran S, et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis: overview and introduction to the full supplement publication. Endoscopy. 2013; 45:51–59. PMID: 23212726.

34. Guidelines for establishment of gastrointestinal endoscopy areas. Gastrointest Endosc. 1991; 37:661–662. PMID: 1756938.
35. Lee TH, Yoon JY, Paik CN, Choi HS, Jang JY. Updates on the facilities, procedures, and performance of the accredited endoscopy unit. Clin Endosc. 2019; 52:431–442. PMID: 31591280.

36. Mayhew D, Mendonca V, Murthy BV. A review of ASA physical status: historical perspectives and modern developments. Anaesthesia. 2019; 74:373–379. PMID: 30648259.
37. ASGE Standards of Practice Committee. Acosta RD, Abraham NS, Chandrasekhara V, Chathadi KV, Early DS, et al. The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointest Endosc. 2016; 83:3–16. PMID: 26621548.

38. Rizk MK, Sawhney MS, Cohen J, Pike IM, Adler DG, Dominitz JA, et al. Quality indicators common to all GI endoscopic procedures. Gastrointest Endosc. 2015; 81:3–16. PMID: 25480102.

39. Zuckerman MJ, Hirota WK, Adler DG, Davila RE, Jacobson BC, Leighton JA, et al. ASGE guideline: the management of low-molecular-weight heparin and nonaspirin antiplatelet agents for endoscopic procedures. Gastrointest Endosc. 2005; 61:189–194. PMID: 15729224.

40. Leslie K, Allen ML, Hessian EC, Peyton PJ, Kasza J, Courtney A, et al. Safety of sedation for gastrointestinal endoscopy in a group of university-affiliated hospitals: a prospective cohort study. Br J Anaesth. 2017; 118:90–99. PMID: 28039246.
41. Tetzlaff JE, Maurer WG. Preprocedural assessment for sedation in gastrointestinal endoscopy. Gastrointest Endosc Clin N Am. 2016; 26:433–441. PMID: 27372768.

42. Vargo JJ 2nd. Sedation-related complications in gastrointestinal endoscopy. Gastrointest Endosc Clin N Am. 2015; 25:147–158. PMID: 25442964.

43. ASGE Standards of Practice Committee. Early DS, Lightdale JR, Vargo JJ 2nd, Acosta RD, Chandrasekhara V, et al. Guidelines for sedation and anesthesia in GI endoscopy. Gastrointest Endosc. 2018; 87:327–337. PMID: 29306520.

44. Harris ZP, Liu J, Saltzman JR. Quality assurance in the endoscopy suite: sedation and monitoring. Gastrointest Endosc Clin N Am. 2016; 26:553–562. PMID: 27372777.
45. ASGE Ensuring Safety in the Gastrointest inal Endoscopy Unit Task Force. Chapman FJ, Cohen J, Cohen LB, Collins J, et al. Guidelines for safety in the gastrointestinal endoscopy unit. Gastrointest Endosc. 2014; 79:363–372. PMID: 24485393.
46. Yom-Tov E, Lebwohl B. Adverse events associated with colonoscopy: an examination of online concerns. BMC Gastroenterol. 2019; 19:207. PMID: 31795939.

47. Rees CJ, Bevan R, Zimmermann-Fraedrich K, Rutter MD, Rex D, Dekker E, et al. Expert opinions and scientific evidence for colonoscopy key performance indicators. Gut. 2016; 65:2045–2060. PMID: 27802153.

48. Kaminski MF, Thomas-Gibson S, Bugajski M, Bretthauer M, Rees CJ, Dekker E, et al. Performance measures for lower gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy. 2017; 49:378–397. PMID: 28268235.

49. Wong MC, Ching JY, Chan VC, Lam TY, Luk AK, Tang RS, et al. Determinants of bowel preparation quality and its association with adenoma detection: a prospective colonoscopy study). Medicine (Baltimore). 2016; 95:e2251. PMID: 26765402.
50. Baker FA, Mari A, Nafrin S, Suki M, Ovadia B, Gal O, et al. Predictors and colonoscopy outcomes of inadequate bowel cleansing: a 10-year experience in 28,725 patients. Ann Gastroenterol. 2019; 32:457–462. PMID: 31474791.

51. Rembacken B, Hassan C, Riemann JF, Chilton A, Rutter M, Dumonceau JM, et al. Quality in screening colonoscopy: position statement of the European Society of Gastrointestinal Endoscopy (ESGE). Endoscopy. 2012; 44:957–968. PMID: 22987217.

52. Rex DK, Schoenfeld PS, Cohen J, Pike IM, Adler DG, Fennerty MB, et al. Quality indicators for colonoscopy. Gastrointest Endosc. 2015; 81:31–53. PMID: 25480100.

53. Vavricka SR, Sulz MC, Degen L, Rechner R, Manz M, Biedermann L, et al. Monitoring colonoscopy withdrawal time significantly improves the adenoma detection rate and the performance of endoscopists. Endoscopy. 2016; 48:256–262. PMID: 26808396.

54. Lee RH, Tang RS, Muthusamy VR, Ho SB, Shah NK, Wetzel L, et al. Quality of colonoscopy withdrawal technique and variability in adenoma detection rates (with videos). Gastrointest Endosc. 2011; 74:128–134. PMID: 21531410.

55. Wierzbicki PM, Adrych K, Kartanowicz D, Stanislawowski M, Kowalczyk A, Godlewski J, et al. Underexpression of LATS1 TSG in colorectal cancer is associated with promoter hypermethylation. World J Gastroenterol. 2013; 19:4363–4373. PMID: 23885148.
56. Patel VD, Thompson WK, Lapin BR, Goldstein JL, Yen EF. Screening colonoscopy withdrawal time threshold for adequate proximal serrated polyp detection rate. Dig Dis Sci. 2018; 63:3084–3090. PMID: 29974376.

57. Patel NC, Islam RS, Wu Q, Gurudu SR, Ramirez FC, Crowell MD, et al. Measurement of polypectomy rate by using administrative claims data with validation against the adenoma detection rate. Gastrointest Endosc. 2013; 77:390–394. PMID: 23199647.

58. Williams JE, Holub JL, Faigel DO. Polypectomy rate is a valid quality measure for colonoscopy: results from a national endoscopy database. Gastrointest Endosc. 2012; 75:576–582. PMID: 22341104.

59. Kaminski MF, Thomas-Gibson S, Bugajski M, Bretthauer M, Rees CJ, Dekker E, et al. Performance measures for lower gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) quality improvement initiative. United European Gastroenterol J. 2017; 5:309–334.

60. Baxter NN, Sutradhar R, Forbes SS, Paszat LF, Saskin R, Rabeneck L. Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology. 2011; 140:65–72. PMID: 20854818.

61. Gingold-Belfer R, Boltin D, Sneh-Arbib O, Comaneshter D, Cohen A, Flugelman A, et al. Association between polyp detection rate and post-colonoscopy cancer among patients undergoing diagnostic colonoscopy. Clin Gastroenterol Hepatol. 2021; 19:202–204. PMID: 31712082.

62. Rees CJ, Thomas Gibson S, Rutter MD, Baragwanath P, Pullan R, Feeney M, et al. UK key performance indicators and quality assurance standards for colonoscopy. Gut. 2016; 65:1923–1929. PMID: 27531829.

63. Muscarella LF. Risk of transmission of carbapenem-resistant Enterobacteriaceae and related “superbugs” during gastrointestinal endoscopy. World J Gastrointest Endosc. 2014; 6:457–474. PMID: 25324917.
64. Kovaleva J, Peters FT, van der Mei HC, Degener JE. Transmission of infection by flexible gastrointestinal endoscopy and bronchoscopy. Clin Microbiol Rev. 2013; 26:231–254. PMID: 23554415.

65. ASGE Quality Assurance in Endoscopy Committee. Calderwood AH, Day LW, Muthusamy VR, Collins J, Hambrick RD 3rd, et al. ASGE guideline for infection control during GI endoscopy. Gastrointest Endosc. 2018; 87:1167–1179. PMID: 29573782.

66. Beilenhoff U, Biering H, Blum R, Brljak J, Cimbro M, Dumonceau JM, et al. Reprocessing of flexible endoscopes and endoscopic accessories used in gastrointestinal endoscopy: position statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology Nurses and Associates (ESGENA). Update 2018. Endoscopy. 2018; 50:1205–1234. PMID: 30458567.
67. Iwakiri R, Tanaka K, Gotoda T, Oka S, Ohtsuka T, Sakata Y, et al. Guidelines for standardizing cleansing and disinfection of gastrointestinal endoscopes. Dig Endosc. 2019; 31:477–497. PMID: 31241788.

68. Reprocessing Guideline Task Force. Petersen BT, Cohen J, Hambrick RD 3rd, Buttar N, Greenwald DA, et al. Multisociety guideline on reprocessing flexible GI endoscopes: 2016 update. Gastrointest Endosc. 2017; 85:282–294. PMID: 28069113.

69. Son BK, Kim BW, Kim WH, Myung DS, Cho YS, Jang BI, et al. Korean Society of Gastrointestinal Endoscopy Guidelines for endoscope reprocessing. Clin Endosc. 2017; 50:143–147. PMID: 28301923.

70. Mehta PP, Kochhar G, Albeldawi M, Kirsh B, Rizk M, Putka B, et al. Capnographic monitoring in routine EGD and colonoscopy with moderate sedation: a prospective, randomized, controlled trial. Am J Gastroenterol. 2016; 111:395–404. PMID: 26902229.

71. Aldrete JA. The post-anesthesia recovery score revisited. J Clin Anesth. 1995; 7:89–91. PMID: 7772368.

72. Chung F. Are discharge criteria changing? J Clin Anesth. 1993; 5(6 Suppl 1):64S–68S. PMID: 8292372.

73. Rudner R, Jalowiecki P, Kawecki P, Gonciarz M, Mularczyk A, Petelenz M. Conscious analgesia/sedation with remifentanil and propofol versus total intravenous anesthesia with fentanyl, midazolam, and propofol for outpatient colonoscopy. Gastrointest Endosc. 2003; 57:657–663. PMID: 12709693.

74. Park HJ, Son BK, Koo HS, Kim BW. Preparation, evaluation, and recovery before and after conscious sedative endoscopy. Korean J Gastroenterol. 2017; 69:59–63. PMID: 28135792.

75. American Association for Study of Liver Diseases. American College of Gastroenterology. American Gastroenteroogical Association Institute. American Society for Gastrointestinal Endoscopy. Society for Gastroenterology Nurses and Associates. Vargo JJ, et al. Multisociety sedation curriculum for gastrointestinal endoscopy. Gastrointest Endosc. 2012; 76:e1–25. PMID: 22624793.

76. Zhang W, Zhu Z, Zheng Y. Effect and safety of propofol for sedation during colonoscopy: a meta-analysis. J Clin Anesth. 2018; 51:10–18. PMID: 30059837.

77. Sahinovic MM, Struys MM, Absalom AR. Clinical pharmacokinetics and pharmacodynamics of propofol. Clin Pharmacokinet. 2018; 57:1539–1558. PMID: 30019172.

SUPPLEMENTARY MATERIALS
Supplementary Tables 1–6 and Supplementary Data can be found via https://doi.org/10.4174/astr.2021.100.3.154.
Supplementary Table 1
Recommended items for assessment of training and competence of colonoscopists
Supplementary Table 2
Recommended items for assessment of facilities and equipment
Supplementary Table 3
Recommended items for assessment of procedure quality assurance
Supplementary Table 4
Recommended items for assessment of performance quality and auditable outcomes
Supplementary Table 5
Recommended items for assessment of infection prevention and disinfection of equipment
Supplementary Table 6
Recommended items for assessment of sedation and recovery of the patient




 PDF
PDF Citation
Citation Print
Print







 XML Download
XML Download