Abstract
Purpose
Initial conservative treatment with selective endovascular or surgical intervention has shown successful outcomes in the treatment of spontaneous isolated superior mesenteric artery dissection (SISMAD). However, the benefits of antithrombotic therapy as a part of conservative treatment have not been clarified. This study aimed to investigate the clinical course of SISMAD patients and determine differences in clinical outcomes between the antithrombotic and no-antithrombotic groups.
Methods
We retrospectively reviewed 79 cases of SISMAD that were treated conservatively from January 2004 to December 2019 at Chonnam National University Hospital. Clinical outcomes, including the length of hospital stay, pain resolution time, image remodeling, and maximal remodeling time, were compared between the antithrombotic and no-antithrombotic groups.
Results
There were 30 patients in the no-antithrombotic group and 49 patients in the antithrombotic group. There was no significant difference in clinical characteristics between the 2 groups, except for dyslipidemia (P = 0.011). The follow-up period (32.6 months vs. 14.6 months, P = 0.009) and imaging follow-up period (31.6 months vs. 13.9 months, P = 0.011) were longer in the antithrombotic group than in the no-antithrombotic group. The length of hospital stay (5.1 days vs. 7.7 days, P = 0.002) was significantly shorter in the no-antithrombotic group than in the antithrombotic group because patients in the antithrombotic group required longer hospitalization for warfarin titration.
Spontaneous isolated superior mesenteric artery dissection (SISMAD) is a rare mesenteric arterial disorder [123456789]. However, its incidence is increasing with increased diagnosis due to the development and widespread application of CT [13567101112]. Some authors have suggested connective tissue disorders as a possible cause of SISMAD [113], but the exact underlying cause of this disorder is unclear [8914].
Treatment of SISMAD includes conservative treatment, endovascular intervention, and surgical intervention. Although it is unclear whether initial conservative treatment or endovascular treatment is better on long-term follow-up [14], recent studies have shown successful outcomes with initial conservative treatment followed by selective endovascular or surgical treatment [2415]. Conservative treatment consists of bowel rest, pain and blood pressure control, and parenteral nutrition with or without antithrombotic therapy. There are studies showing successful outcomes with antithrombotic therapy [41116], but some studies [512] have also shown successful outcomes without antithrombotic therapy. Relatively few studies have investigated outcomes comparing the antithrombotic and no-antithrombotic groups; they revealed no significant differences in clinical outcomes [31417]. In this study, we investigated the clinical course of SISMAD patients who were treated conservatively to evaluate differences in clinical outcomes between the antithrombotic and no-antithrombotic groups. We also aimed to identify clinical characteristics related to SISMAD remodeling.
This study was approved by the Institutional Review Board of Chonnam National University Hospital (No. CNUH 2020-171). The requirement for informed consent was waived because of the retrospective nature of the study.
All patients with SISMAD who were diagnosed at our hospital between January 2004 and December 2019 and initially treated conservatively were included. Patients who initially received endovascular or surgical intervention for the treatment of SISMAD were excluded from this study. Patients without at least 3 months of outpatient follow-up and those in whom the latest follow-up imaging test was performed 3 or more months after the initial diagnostic imaging test were excluded. Patients with aortic dissection or other mesenteric arterial disease and those with a history of abdominal trauma were also excluded.
A total of 79 patients were included in this study. Their medical records were retrospectively reviewed to identify clinical characteristics such as age, sex, comorbidities, clinical manifestations, drug history, and imaging changes during follow-up. If the patient was symptomatic on initial presentation, the time interval between the onset of pain and beginning of treatment, and the time interval between the beginning of treatment and resolution of pain were assessed. Resolution of pain was defined as there being neither resting pain nor postprandial pain.
On contrast-enhanced CT, patients were diagnosed with SISMAD when arterial dissection of the superior mesenteric artery was observed without aortic or other mesenteric dissection. The categorization of SISMAD described by Yun et al. [2] was used to classify SISMAD. The length of dissection was recorded as the fractional ratio of the entire length of dissection to the total length of the superior mesenteric artery. The fractional ratio of dissection length was categorized into 3 groups: fractional ratio less than 1/3 was defined as length-A; fractional ratio equal to or more than 1/3 but less than 2/3 was length-B; and fractional ratio equal to or more than 2/3 was length-C.
If SISMAD was detected incidentally without symptoms, the patient was followed up on an outpatient basis with routine contrast-enhanced CT and was examined for abdominal symptoms relevant to SISMAD. Symptomatic patients diagnosed with SISMAD were treated conservatively, except for those who were suspected to have peritonitis or intestinal ischemia on physical examination and CT scans. Patients with suspected peritonitis or intestinal ischemia, and those who had aggravating or unrelievable abdominal pain despite conservative treatment were indicated for endovascular intervention or surgery.
Conservative treatment consisted of fasting until there was no abdominal pain without pain control. Feeding was carefully advanced from water to a regular diet while monitoring for recurrence of abdominal symptoms. Intravenous fluids and parenteral nutrition were administered to prevent dehydration and support nutrition.
Antithrombotic treatment included anticoagulation therapy consisting of intravenous heparin and warfarin, and included antiplatelet agents such as aspirin, clopidogrel, or cilostazol. The choice of antithrombotic combination was left to the discretion of the doctor. From January 2004 to December 2016, antithrombotic treatment was applied to newly diagnosed patients. However, from January 2017 to December 2019, routine antithrombotic treatment was abandoned for newly diagnosed patients due to the recognition of the possible ineffectiveness of antithrombotic treatment in improving clinical outcomes in these patients. In patients who were already taking antithrombotic medications due to underlying diseases at the time of diagnosis, the antithrombotic medications were continued as prescribed.
If patients were able to tolerate a regular diet without abdominal symptoms after conservative treatment, the patient was discharged. Routine follow-up was performed on an outpatient basis.
During follow-up, recurrence of symptoms such as postprandial pain was monitored, and imaging follow-up with CT angiography was performed every 3 months for the first 6 months, every 6 months for the next 6 months, and then once annually, to monitor changes in the angiographic features of SISMAD.
Follow-up CT scans were compared with previous CT scans for changes in the extent of dissection, true lumen stenosis, false lumen thrombosis, branch artery dissection, aneurysmal change of the dissected lesion, and other mesenteric artery disease.
In the classification of SISMAD on the follow-up results, complete remodeling was defined as the presence of no remnant dissection or aneurysmal change and normalization of superior mesenteric artery morphology. Incomplete remodeling was defined as an improved state of true lumen stenosis without dissection-type change. Type change was defined as the categorization of SISMAD being different on follow-up imaging than that on initial diagnosis. No change was defined as the follow-up images of SISMAD remaining stationary without significant changes in true lumen stenosis and morphology. Maximal remodeling was defined as complete or incomplete remodeling that was stationary without morphological changes for 2 consecutive CT scans. Maximal remodeling time, defined as the time interval between initial diagnosis and maximal remodeling, was also measured. When patients were sufficiently followed up and reached maximal remodeling, antithrombotic medications were stopped, except in those patients who required antithrombotic treatment for other medical reasons.
To dichotomize imaging follow-up results, the complete and incomplete remodeling groups were assigned to a ‘remodeling group’ and the no-change group to a ‘no remodeling group.’ Only 1 patient showed a type change, which was from type-2b SISMAD at initial presentation to type-2a SISMAD at the 1-month follow-up, and it remained stationary after that for 2 years. We assigned this patient to the no remodeling group because even though there was a type change during follow-up, true lumen stenosis did not show improvement.
There was no treatment failure or complication resulting in delayed endovascular or surgical intervention in all patients who were initially treated conservatively. Variables such as the time interval between the beginning of treatment and pain resolution, the length of hospital stay, remodeling during follow-up imaging, and maximal remodeling time were used to compare clinical outcomes between the antithrombotic and noantithrombotic groups.
Continuous variables are given as mean and standard deviation or range, and nominal variables are given as numbers and percentages. The chi-square or Fisher exact test was used for nominal variables, and the Mann-Whitney test was used for continuous variables to compare differences between the antithrombotic and no-antithrombotic groups, and between the remodeling and no remodeling groups. Multivariate logistic regression analysis was performed to identify variables that were independently associated with image remodeling. Pearson correlation analysis was performed to identify the correlation between the follow-up period and maximal remodeling time. Statistical significance was set at P < 0.05. IBM SPSS Statistics version 23 (IBM Corp., Armonk, NY, USA) was used to perform all statistical analyses.
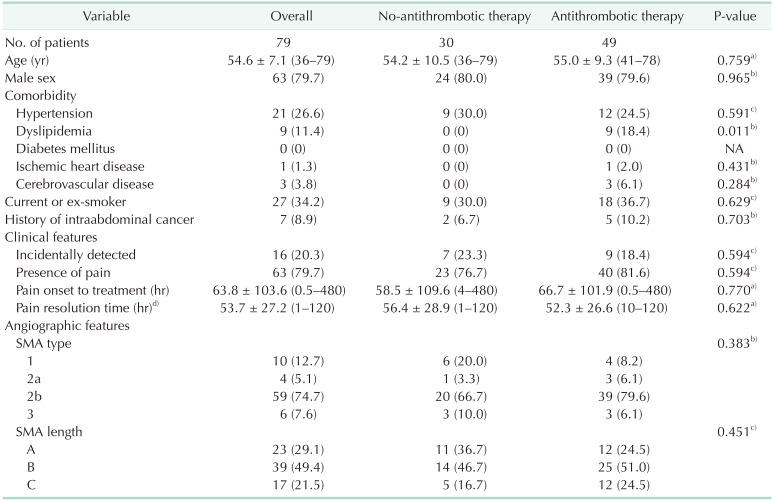
Between January 2004 and December 2019, 130 patients were diagnosed with SISMAD. Of them, 15 patients initially underwent endovascular intervention and 5 patients underwent surgical intervention. One hundred and 10 patients were initially treated conservatively, and this study included 79 patients (mean age, 54.6 ± 7.1 years; male, 79.7%) who met the inclusion criteria. Table 1 shows the clinical characteristics that were assessed at initial presentation. Hypertension (21, 26.6%), dyslipidemia (9, 11.4%), diabetes mellitus (0), ischemic heart disease (1, 1.3%), cerebrovascular disease (3, 3.8%), smoking status (current or ex-smoker; 27, 34.2%), and history of intraabdominal cancer (7, 8.9%) were assessed. Sixteen patients (20.3%) were diagnosed incidentally, and 63 patients (79.7%) were symptomatic with pain. Symptoms other than pain and the location of pain are summarized in Supplementary Table 1. Angiographic features at initial diagnosis showed 10 type-1 patients (12.7%), 4 type-2a patients (5.1%), 59 type-2b patients (74.7%), and 6 type-3 patients (7.6%). According to length distribution, there were 23 length-A patients (29.1%), 39 length-B patients (49.4%), and 17 length-C patients (21.5%).
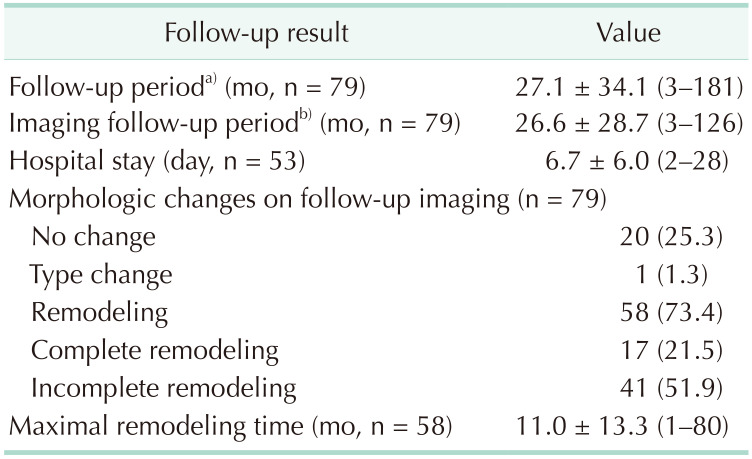
Clinical follow-up data after conservative treatment are summarized in Table 2. All 79 patients were followed up for at least 3 months, with a median follow-up of 27.1 months (range, 3–181 months). All patients underwent follow-up CT angiography for at least 3 months after initial diagnosis, with a mean imaging follow-up duration of 26.6 months (range, 3–126 months). Of the 79 patients, 56 patients who were symptomatic at initial diagnosis were hospitalized, with a mean length of hospital stay of 6.7 days (range, 2–28 days). Morphologic changes were also seen during follow-up, with 58 patients (73.4%) showing remodeling, of which 17 (21.5%) showed complete remodeling. Twenty patients (25.3%) showed no change, and in only 1 patient, change from type 2b to type 2a occurred. Mean maximal remodeling time was 11 months (range, 1–80 months). Of the 79 patients, there was no treatment failure or complication after conservative treatment that resulted in later endovascular or surgical interventions. In the antithrombotic group, there were no remarkable antithrombotic-related complications.
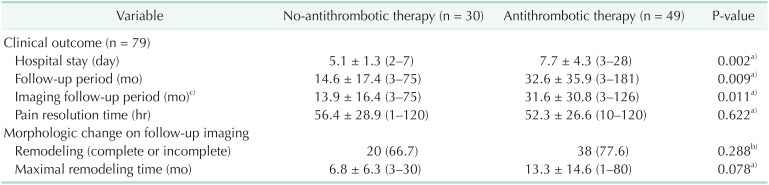
The no-antithrombotic group contained 30 patients, and the antithrombotic group contained 49 patients. As described in Table 1, the main clinical characteristic different between the 2 groups was dyslipidemia (P = 0.011). Other than dyslipidemia, there was no significant difference in clinical characteristics. Clinical outcomes and morphologic changes in the follow-up CT scans are summarized in Table 3. The follow-up period (14.6 months vs. 32.6 months, P = 0.009) and imaging follow-up period (13.9 months vs. 31.6 months, P = 0.011) were longer in the antithrombotic group than in the no-antithrombotic group. Pain resolution time (56.4 hours vs. 52.3 hours, P = 0.622) between the 2 groups did not show a significant difference. The no-antithrombotic group had a significantly shorter length of hospital stay (5.1 days vs. 7.7 days, P = 0.002) than the antithrombotic group. During follow-up, remodeling of SISMAD (66.5% vs. 77.6%, P = 0.288) did not show a significant difference between the groups. Although not statistically significant, the no-antithrombotic group tended to have a shorter maximal remodeling time (6.8 months vs. 13.3 months, P = 0.078). To clarify the reason for the shorter maximal remodeling time in the no-antithrombotic group, Pearson correlation analysis was conducted between maximal remodeling time and follow-up duration for each group. Both the no-antithrombotic group (r = 0.875, P < 0.001) and antithrombotic group (r = 0.286, P = 0.081) showed a positive correlation between maximal remodeling time and follow-up period.
Of the 49 patients in the antithrombotic group, 6 (12.2%) were receiving anticoagulation treatment consisting of intravenous heparin and warfarin, 39 (79.6%) were receiving antiplatelet medication, and 4 (8.2%) were receiving both anticoagulation and antiplatelet treatment. The mean duration of antithrombotic treatment was 15.2 months (range, 3–106 months).
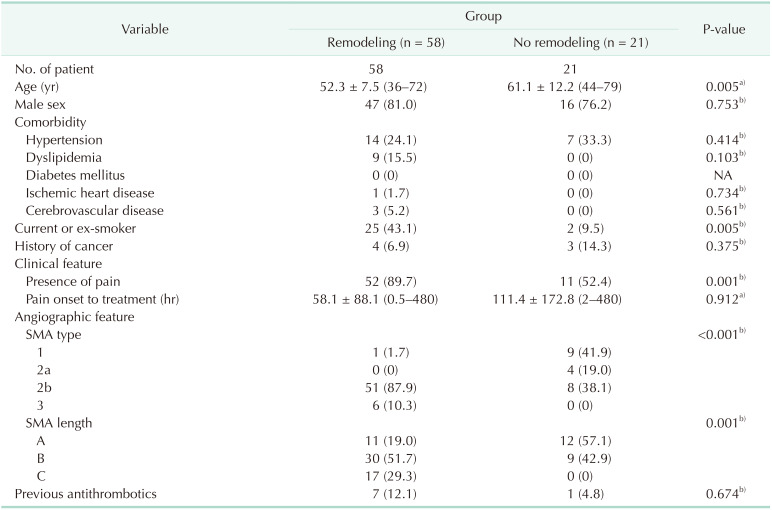
In Table 4, we investigated clinical characteristics, including angiographic features, to determine predictors associated with SISMAD remodeling that could be obtained at the time of diagnosis. The remodeling group showed a statistically younger mean age (52.3 years vs. 61.1 years, P = 0.005) and a larger proportion of current or ex-smokers (43.1% vs. 9.5%, P = 0.005) than the non-remodeling group. The remodeling group was associated with more frequent presence of pain compared to the no remodeling group (89.7% vs. 52.4%, P = 0.001). With respect to angiographic features, the remodeling group showed less frequent type-1 change and more frequent type-2b and type-3 changes (1.7% vs. 41.9%, 87.9% vs. 38.1%, and 10.3% vs. 0%; P < 0.001). With respect to SISMAD length, the remodeling group had more frequent length-B and length-C cases (76.9% vs. 42.9% and 29.3% vs. 0%, P = 0.001).
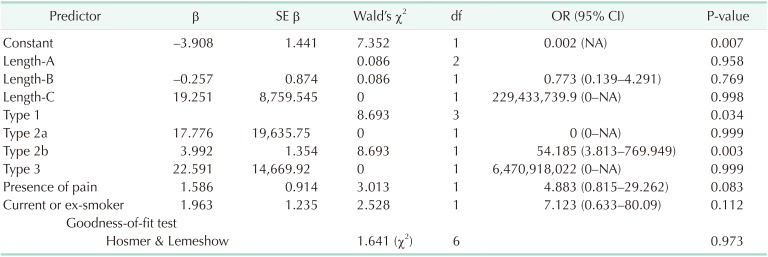
Age, dissection length, dissection type, current or exsmoker, and presence of pain were the variables included in the multivariate logistic regression analysis, and the final multivariate model is summarized in Table 5. Type-2b SISMAD cases were more likely to show remodeling compared to type-1 cases, and the odds ratio were 52.185 (= e3.992, P = 0.003). Other factors were not found to be independent risk factors for remodeling in the analysis. As a goodness-of-fit test, the Hosmer-Lemeshow test showed a P-value of 0.973, implying that there was good fit.
There were 63 symptomatic patients in the entire study group who were divided into the no-antithrombotic group (n = 23) and the antithrombotic group (n = 40). There were no significant differences in clinical characteristics other than dyslipidemia (0 vs. 17.5, P = 0.041) between the 2 groups. As shown in Supplementary Table 2, similar to the entire cohort (n = 79) of symptomatic patients, the no-antithrombotic group had a significantly shorter hospital stay (7.7 days vs. 5.1 days, P = 0.002), follow-up period (27.22 months vs. 10.6 months, P = 0.021), and imaging follow-up period (29.4 months vs. 10.4 months, P = 0.022). Remodeling (82.6% vs. 82.5%, P = 0.991) did not show a significant difference. The mean maximal remodeling time was shorter in the no-antithrombotic group (5.5 months vs. 11.8 months, P = 0.147) but was not statistically significant. In Supplementary Table 3, clinical characteristics are compared to investigate predictors related to remodeling in symptomatic SISMAD patients. The results were similar to the entire cohort, except that the dissection length showed marginal significance (P = 0.053). Age, current or ex-smoker, dissection type, and dissection length were included in the multivariate logistic regression analysis, and the final model is summarized in Supplementary Table 4. Similar to the entire cohort, type-2b dissection was more likely to show remodeling compared to type-1 dissection, and the odds ratio were 46.562 (= e3.841, P = 0.003).
The aim of this study was to identify the difference in clinical outcomes between the no-antithrombotic and antithrombotic groups that were treated conservatively for SISMAD. Furthermore, we sought to determine the clinical characteristics associated with remodeling of SISMAD.
In SISMAD patients without bowel ischemia, conservative treatment is currently suggested as initial treatment [341518]. Bowel rest, pain control, blood pressure control, and parenteral nutrition are the main components of conservative treatment. However, antithrombotic therapy is still controversial. Some authors have suggested routine antithrombotic therapy as a part of conservative treatment based on concerns of thrombus formation and distal embolization [411]. A previous study proposed only selective antithrombotic therapy for severely compromised blood flow, based on the idea that antithrombotic therapy could increase the risk of intramural hemorrhage at the dissected lesion [310]. Other studies have suggested conservative treatment without antithrombotic therapy [512]. Few studies have compared antithrombotic therapy and noantithrombotic therapy, and they revealed no significant difference in clinical outcomes between the 2 groups [317]. In a meta-analysis of 35 articles [14], antithrombotic treatment for SISMAD regardless of symptoms did not yield beneficial outcomes, and its use was not recommended. Our results show that not only did antithrombotic therapy yield no clinical benefits but also that the no-antithrombotic group showed significantly shorter length of hospital stay and tended to have shorter maximal remodeling time.
The reason for the shorter length of hospital stay in the no-antithrombotic group was because patients in the antithrombotic group treated with anticoagulation required longer hospitalization for warfarin titration. Even if the antithrombotic group was divided into an antithrombotic-without anticoagulation group and an antithrombotic-with anticoagulation group, the length of hospital stay tended to be longer in the antithrombotic-with anticoagulation group (10.7 days vs. 6.7 days, P = 0.067). Even though novel oral anticoagulants (NOACs) are convenient and do not require titration, the National Health Insurance does not cover NOACs in these circumstances, limiting prescription.
With respect to maximal remodeling time, Pearson correlation analysis revealed positive correlation between maximal remodeling time and outpatient follow-up duration. We believe that this is due to several reasons. Because the no-antithrombotic strategy was started in the later period of the study, the follow-up duration was naturally shorter in this group than in the antithrombotic group. In addition, we think that follow-up losses were more frequent in the no-antithrombotic group because there were no medications prescribed regularly, and only routine follow-up imaging. Due to our follow-up protocol, patients were scheduled for 3 visits and imaging follow-up during the first year, which was more frequent than the annual follow-up after the first year. During these relatively frequent visits, patients could have been prematurely diagnosed with maximal remodeling due to the relative frequency of imaging studies. In summary, the no-antithrombotic group consisted of patients with shorter follow-up durations due to later recruitment and relatively frequent follow-up loss. Patients with shorter follow-up duration may have experienced premature diagnosis of maximal remodeling. Unlike most other studies, we applied a minimum follow-up duration of 3 months, excluding 31 patients with less than 3 months of follow-up. We believe that to evaluate the relationship between morphological change and antithrombotic treatment, only long-term follow-up patients should be included. Therefore, further long-term follow-up studies are needed to identify the true relationship between maximal remodeling time and antithrombotic treatment.
Our standard of choosing initial treatment was to opt for conservative treatment unless there was evidence of intestinal ischemia or peritonitis. Persistent or aggravating pain despite conservative treatment, and evidence of intestinal ischemia or peritonitis at the initial presentation were the criteria for endovascular or surgical intervention. Although these treatment algorithms were not very different from those in other studies [341519], our results showed 0% treatment failure or late complications after conservative treatment that necessitated additional endovascular or surgical interventions, which is relatively low compared to previous studies (0%–22%) [235611121518]. A review of 51 articles reported that 18.1% of conservative treatment resulted in additional treatment [20]. Heo et al. [3] reported 2 cases of late complications resulting in small bowel resection due to small bowel stricture at 1.5 months and 3.8 months after diagnosis, which is shorter than our mean follow-up period of the no-antithrombotic group (14.6 months; range, 3–75 months). The initial intervention rates in our study were 11.5%, 3.8%, and 15.4% (endovascular, surgery, and overall), which were not so different from those of previous studies, which were 3%–16%, 2–9%, and 6%–23% [234161920]. This result may imply that careful selection of initial treatment in SISMAD patients might make it possible to lower treatment failure and late complications that resulted in additional interventions.
In this study, we defined a new concept of maximal remodeling, which could be applied to various existing SISMAD classification methods to aid in expressing the degree of follow-up image changes and to help determine follow-up duration. There are no studies that directly mention termination of outpatient follow-up. Han et al. [11] mentioned terminating follow-up of incomplete remodeling that showed no progression at 12 months of follow-up CT angiography. We believe that future studies on SISMAD could incorporate the concept of maximal remodeling of SISMAD to evaluate and compare changes in imaging, which may also help decide the termination of follow-up.
Our univariate analysis of factors associated with positive remodeling revealed younger age, smoking history, presence of pain, longer dissection, and dissection type 2b or type 3, and vice versa for negative remodeling. To explain this phenomenon, we hypothesized that there could be a different pathophysiology between symptomatic younger patients with smoking history showing long type-2b or type-3 dissection and asymptomatic older patients without a smoking history showing short type-1 or type-2a dissection, which affects the remodeling of superior mesenteric artery dissection. Since the underlying cause of this disease is still unclear, further studies are needed to clarify these observations.
Factors associated with frequent remodeling reported in previous studies [31516] were type-3 lesions [3], type-2 lesions [15], presence of symptoms, and absence of false lumen. Factors associated with no change were type-1 lesions [315]. Our multivariate logistic regression analysis revealed that type- 2b SISMAD was associated with more frequent remodeling, and type-1 SISMAD was associated with no change in follow-up images, which is similar to results from previous studies. These factors could be considered at the beginning of treatment as useful information for discussing treatment modalities and outcomes with patients.
There were several limitations to this study. As a retrospective study, we could not randomize patients into different treatment groups. Because there is no widely accepted treatment guideline for SISMAD, the antithrombotic combination was chosen based on the doctors' discretion. Baseline characteristics including comorbidities, clinical features, and angiographic features did not differ except for dyslipidemia between the 2 groups, but there were possible selection biases due to the inclusion of 6 patients in the antithrombotic group although they were taking antithrombotic medications for their underlying diseases. Although our study included a relatively large number of patients compared to other studies, we could not compare anticoagulation and antiplatelet treatment separately as a subgroup of antithrombotic therapy.
In conclusion, our study showed that in patients with SISMAD, conservative treatment without antithrombotic therapy may have clinical benefits such as decreased length of hospital stay due to not requiring warfarin titration compared with conservative treatment with antithrombotic therapy. Conservative treatment has been shown to be a successful treatment modality in patients without intestinal ischemia and with a low complication rate. Therefore, in patients with SISMAD, conservative treatment with antithrombotic therapy may have no beneficial effect compared with conservative treatment without antithrombotic therapy.
References
1. Kim YW. Current understandings of spontaneous isolated superior mesenteric artery dissection. Vasc Specialist Int. 2016; 32:37–43. PMID: 27386450.

2. Yun WS, Kim YW, Park KB, Cho SK, Do YS, Lee KB, et al. Clinical and angiographic follow-up of spontaneous isolated superior mesenteric artery dissection. Eur J Vasc Endovasc Surg. 2009; 37:572–577. PMID: 19208448.

3. Heo SH, Kim YW, Woo SY, Park YJ, Park KB, Kim DK. Treatment strategy based on the natural course for patients with spontaneous isolated superior mesenteric artery dissection. J Vasc Surg. 2017; 65:1142–1151. PMID: 28216343.

4. Cho BS, Lee MS, Lee MK, Choi YJ, Kim CN, Kang YJ, et al. Treatment guidelines for isolated dissection of the superior mesenteric artery based on follow-up CT findings. Eur J Vasc Endovasc Surg. 2011; 41:780–785. PMID: 21333559.

5. Kim H, Park H, Park SJ, Park BW, Hwang JC, Seo YW, et al. Outcomes of spontaneous isolated superior mesenteric artery dissection without antithrombotic use. Eur J Vasc Endovasc Surg. 2018; 55:132–137. PMID: 29229279.

6. Park UJ, Kim HT, Cho WH, Kim YH, Miyata T. Clinical course and angiographic changes of spontaneous isolated superior mesenteric artery dissection after conservative treatment. Surg Today. 2014; 44:2092–2097. PMID: 24496981.

7. Shiraki H, Kasamoto M, Yasutomi M, Kaji S, Akutsu K, Furukawa Y, et al. Clinical features of spontaneous isolated dissection of abdominal visceral arteries. J Clin Med Res. 2020; 12:13–17. PMID: 32010417.

8. Le TB, Jeon YS, Hong KC, Cho SG, Park KM. Spontaneous dissections of multiple visceral arteries: an extremely rare case. Ann Surg Treat Res. 2017; 92:225–229. PMID: 28382296.

9. Lee MK, Cho BS, Han HY, Jung IM, Park SH, Kim CN, et al. Isolated dissection of superior mesenteric artery: study on the treatment guidelines. J Korean Surg Soc. 2010; 79:64–70.

10. Wang J, He Y, Zhao J, Yuan D, Xu H, Ma Y, et al. Systematic review and meta-analysis of current evidence in spontaneous isolated celiac and superior mesenteric artery dissection. J Vasc Surg. 2018; 68:1228–1240. PMID: 30126785.

11. Han Y, Cho YP, Ko GY, Seo DW, Kim MJ, Kwon H, et al. Clinical outcomes of anticoagulation therapy in patients with symptomatic spontaneous isolated dissection of the superior mesenteric artery. Medicine (Baltimore). 2016; 95:e3480. PMID: 27100453.

12. Min SI, Yoon KC, Min SK, Ahn SH, Jae HJ, Chung JW, et al. Current strategy for the treatment of symptomatic spontaneous isolated dissection of superior mesenteric artery. J Vasc Surg. 2011; 54:461–466. PMID: 21571493.

13. Hashimoto T, Deguchi J, Endo H, Miyata T. Successful treatment tailored to each splanchnic arterial lesion due to segmental arterial mediolysis (SAM): report of a case. J Vasc Surg. 2008; 48:1338–1341. PMID: 18971044.

14. Ahn S, Mo H, Han A, Min SI, Min SK, Ha J, et al. The use of antithrombotics is not beneficial for conservative management of spontaneous isolated dissection of the superior mesenteric artery: a meta-analysis. Ann Vasc Surg. 2019; 60:415–423. PMID: 31075482.

15. Park YJ, Park KB, Kim DI, Do YS, Kim DK, Kim YW. Natural history of spontaneous isolated superior mesenteric artery dissection derived from follow-up after conservative treatment. J Vasc Surg. 2011; 54:1727–1733. PMID: 21944909.

16. Kimura Y, Kato T, Nagao K, Izumi T, Haruna T, Ueyama K, et al. Outcomes and radiographic findings of isolated spontaneous superior mesenteric artery dissection. Eur J Vasc Endovasc Surg. 2017; 53:276–281. PMID: 28012909.

17. Loeffler JW, Obara H, Fujimura N, Bove P, Newton DH, Zettervall SL, et al. Medical therapy and intervention do not improve uncomplicated isolated mesenteric artery dissection outcomes over observation alone. J Vasc Surg. 2017; 66:202–208. PMID: 28506477.

18. Jang JH, Cho BS, Ahn HY, Lee S, Kim H, Kim CN. Optimal treatment strategy and natural history of isolated superior mesenteric artery dissection based on long-term follow-up CT findings. Ann Vasc Surg. 2020; 63:179–185. PMID: 31626943.

19. Satokawa H, Takase S, Wakamatsu H, Seto Y, Kurosawa H, Yamamoto A, et al. Long-term outcomes of spontaneous isolated superior mesenteric artery dissection. Ann Vasc Dis. 2019; 12:456–459. PMID: 31942202.

20. Kimura Y, Kato T, Inoko M. Outcomes of treatment strategies for isolated spontaneous dissection of the superior mesenteric artery: a systematic review. Ann Vasc Surg. 2018; 47:284–290. PMID: 28751168.

SUPPLEMENTARY MATERIALS
Supplementary Tables 1–4 can be found via https://doi.org/10.4174/astr.2021.100.3.166.
Supplementary Table 1
Comparison of pain location and symptoms other than pain
Supplementary Table 2
Comparison of clinical outcomes and morphologic changes of symptomatic SISMAD patients (n = 63) by antithrombotic use
Supplementary Table 3
Comparison of clinical characteristics and angiographic features among symptomatic patients between remodeling and no remodeling group
Supplementary Table 4
Logistic regression analysis for symptomatic SISMAD remodeling in follow-up imaging




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download