Abstract
Central cord syndrome (CCS) is extremely rare as a direct consequence of generalized epileptic seizure. CCS is associated with hyperextension of the spinal cord and has characteristic radiologic findings including posterior ligamentous injury and prevertebral hyperintensity following magnetic resonance imaging (MRI). We experienced the case of a 25-year-old man who suffered CCS after status epilepticus. Cervical spinal MRI revealed high signal intensity at the C1 level but with no signal or structural changes in other sites. After rehabilitation management, the patient significantly improved on the ASIA (American Spinal Injury Association) motor scale and bladder function. We proposed that epilepsy related CCS may be caused by muscle contractions during generalized seizure, which can induce traction injury of the spinal cord or relative narrowing of spinal canal via transient herniated nucleus pulposus or transient subluxation of vertebra. We also suggest CCS without radiologic findings of trauma has good prognosis compared with other CCS.
Refractory epilepsy is a seizure disorder that does not have remission despite appropriate therapy with anti-epileptic drugs. It is estimated to affect up to 30% of patients with epilepsy.1 Of epilepsy related injuries, face and head injuries are most commonly seen following seizures except minor soft tissue injuries.2 Otherwise, burns and fractures are observed. Spinal cord injury following seizure is usually a complication of vertebral fractures, of which the thoracic vertebra is most commonly involved. The cervical spinal cord injury is rarely caused by seizure itself, but is secondary to the cervical vertebra injury, mainly by falls following seizure or associated with hyperextension in patients with spondylotic canal.3
We report a case of central cord syndrome (CCS) after status epilepticus, in which the patient showed only intramedullary signal change, with no other evidence of trauma or degenerative changes on MRI.
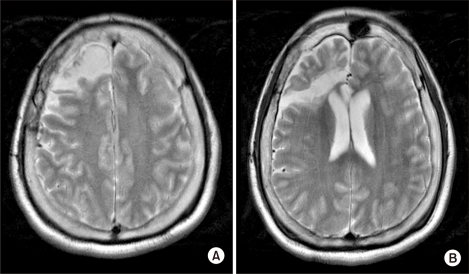
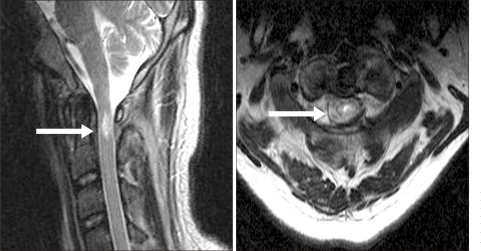
A 25 year-old man was admitted to the emergency department in our hospital due to status epilepticus. He had developed epilepsy at the age of 12 months. Despite taking anti-epileptic medications, the seizures were not controlled. He underwent right temporal lobectomy at the age of 20 (Fig. 1). Although right corpus callosotomy was performed after 2 years, he continued to suffer from seizures. The refractory epilepsy was of a generalized tonic-clonic nature, and occurred 4-5 times a day, each episode lasting 4-5 minutes. The patient could not independently perform activities of daily living because of pre-existing mental retardation, but showed no motor function impairment. He was brought to the emergency department due to status epilepticus. When he arrived at our hospital, he continued to experience generalized tonic-clonic seizures. He was treated with intravenous anti-epileptics and mechanical ventilation was performed after intubation. On hospital Day 3, he regained consciousness and could blink his eyes when directed. However, it was noted that he suffered from complete quadriplegia under the Medical Research Council (MRC) classification for most muscle groups. There were no signs of external trauma on physical examination and no evidence of trauma, such as fracture or dislocation, on plain radiography of the cervical spine (Fig. 2). His condition was diagnosed as Todd's paralysis and further work-up was not considered. On hospital Day 7, motor power showed a total score of 4 on the ASIA motor scale in the lower limbs and 0 in the upper limbs. MRI of the cervical spine was performed and showed high signal intensity in C1 of the cervical spinal cord, without changes in the surrounding tissue or bone (Fig. 3). A diagnosis of CCS was made and steroid pulse therapy was started. On hospital Day 19, the patient was successfully weaned off mechanical ventilation and no respiratory distress was noted. He was transferred to the rehabilitation unit one month after admission. At that time, the motor power of all 4 limbs had a score of 45 on the ASIA motor scale, 13 in the upper limb and 32 in the lower limb. Neurologic level could not be assessed on the ASIA impairment scale because his cognitive impairment precluded the sensory level examination. He scored 9 on the modified Barthel Index (MBI) and managed his bladder with an indwelling catheter. After one month of rehabilitation management, his overall score on the ASIA motor scale improved to 73, 30 in the upper limb and 43 in the lower limb. He was able to stand up from the sitting position. Although he was able to walk with an anterior walker for about 50 meters, moderate assistance of a caregiver was needed. He urinated by valsalva and diaper voiding.
It has been reported that patients diagnosed with epilepsy suffered not only from the psychological and social components of the disorder, but also from physical trauma produced by either paralysis or loss of consciousness during a seizure episode.4 Physical trauma was reported in 3% of the investigated patients diagnosed with epilepsy and fracture risk was two times higher than the general population.5 The spine is the most common epilepsy related fracture site and is usually injured from repeated flexion and extension movements as well as violent muscle contractions that occur during a seizure. Vertebral compression fracture is the most common type of non-traumatic fracture complicating the convulsive seizure.3 It decreases the height of the anterior aspect of the vertebral body sparing its posterior element. Compression fracture is most commonly noted in the thoracic vertebra, and frequently asymptomatic. The epilepsy-related cervical fracture is rare and occurs secondary to the trauma following epilepsy. Kruitbosch et al.6 reported that the incidence of cervical spinal cord injuries in patients with refractory epilepsy is 30-40 times higher than in the normal population. The injuries occur after seizure-related falls, and all patients suffer from simultaneous face or head injuries. Considering the mechanisms of vertebral fracture, the most common site of spinal cord injury in persons with epilepsy is the thoracic spinal cord. The thoracic spinal cord injury occurs as a complication of generalized convulsive seizures and does not usually accompany a traumatic fracture.
It has been documented that CCS results from hyperextension and subsequent cord compression between the enfolded ligamentum flavum and the anterior vertebral osteophyte.7 The younger population appeared to suffer after sustaining trauma to the cervical spine, whereas injuries in the older population resulted from a hyperextension mechanism with underlying cervical spondylosis.8 Characteristic findings of the central cord syndrome were either posterior ligamentous injury or the intramedullary and prevertebral high signal intensity on T2 weighted MRI, both related to spinal cord and surrounding tissue damages.8
We presented the case of cervical spinal cord injury following status epilepticus in a patient with refractory epilepsy. This patient was clinically compatible with the CCS without the evidence of trauma. T2 weighted MRI showed only intramedullary high signal intensity on C1 spinal cord. These were distinct findings from an epilepsy-related spinal cord injury, which is generally caused by the seizure itself or is secondary to trauma during the seizure. In adults, a spinal cord injury without radiologic abnormality is rarely observed. It may occur in relation with a hyperextension injury or a hyperflexion-compression force, which transmits the axial loading caudally. The cord damage occurs as a consequence of traction to the upper part of the injured spinal cord by forward translation along with compression of the lower part of the injured spinal cord segment between a modest bulged disc and the edges of the laminae. In children, spinal cord injury without radiologic abnormality was related to hyperextension injury, when the spinal cord was compressed by a transient vertebral dislocation or disc herniation.9 Therefore, the spinal cord injury in this case may have occurred due to traction of the injured spinal cord segment or due to compression by transient cervical translocation or disc herniation.
The prognostic factors of the functional outcome in the CCS include age, good hand function, initial ASIA motor score, evidence of early motor recovery during rehabilitation, abnormal signal intensity on MRI such as spinal cord edema and hemorrhage, steroid administration at the time of injury, spasticity, presence of an underlying disease and MBI scores at the time of admission. Of these, initial motor score of less than 20 on the ASIA motor scale, as well as the MRI evidence of abnormal signal intensity and steroid administration at the time of injury were the most import predictive factors of functional outcome.7 Similarly, patients with no evidence of fracture or spondylotic changes showed better prognosis than those with signs of cord edema, parenchymal hemorrhage or contusion on MRI.9
Considering the prognostic factors of intramedullary high signal intensity on MRI, which were delayed administration of steroid and the initial motor score of 4 on the ASIA motor scale, the predictive functional outcome of this patient was poor. However, the patient significantly improved motor function, compared with functional recovery reported by Lim et al.10 in patients with traumatic central cord syndrome. Lim et al.10 reported that patients who regained functional independent ambulation scored more than 63.4 on the early ASIA motor scale whereas patients with reflex voiding scored more than 43.3. We believe that his functional recovery was better than other patients with CCS because his injury was confined to one segment without underlying lesions. Although his lower extremity function improved to 43 on the ASIA motor scale, the patient could not ambulate independently. The functional outcome after rehabilitation was thought to be affected by attention and learning deficit with mental retardation. Therefore continuous rehabilitation is needed for independent walking ambulation.
This case showed spinal cord injury caused by epilepsy itself without evidence of trauma or other underlying lesions. It was noted that functional recovery was better than generally observed improvement in patients with central cord syndrome. In conclusion, spinal cord injury should be considered in epilepsy patients showing paralysis after the seizure episode.
References
1. Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000; 342:314–319. PMID: 10660394.

3. Roohi F, Fox A. Burst fracture of the first lumbar vertebra and conus-cauda syndrome complicating a single convulsive seizure: a challenge of diagnosis in the Emergency Department. J Emerg Med. 2006; 31:381–385. PMID: 17046478.

4. Kim YH, Kim HI, Ko DH, Woo YJ, Kim KW, Lee MC, Shin YI, Jang JH. Methodology for assessing and promoting the quality of life in patients with epilepsy. J Korean Acad Rehabil Med. 1996; 20:506–517.
5. Souverein PC, Webb DJ, Petri H, Weil J, Van Staa TP, Egberts T. Incidence of fractures among epilepsy patients: a population-based retrospective cohort study in the General Practice Research Database. Epilepsia. 2005; 46:304–310. PMID: 15679512.

6. Kruitbosch JM, Schouten EJ, Tan IY, Veendrick-Meekes MJ, de Vocht JW. Cervical spinal cord injuries in patients with refractory epilepsy. Seizure. 2006; 15:633–636. PMID: 17070074.

7. Hohl JB, Lee JY, Horton JA, Rihn JA. A novel classification system for traumatic central cord syndrome: the central cord injury scale (CCIS). Spine. 2010; 35:E238–E243. PMID: 20228699.
8. Nowak DD, Lee JK, Gelb DE, Poelstra KA, Ludwig SC. Central cord syndrome. J Am Acad Orthop Surg. 2009; 17:756–765. PMID: 19948700.

9. Tewari MK, Gifti DS, Singh P, Khosla VK, Mathuriya SN, Gupta SK, Pathak A. Diagnosis and prognostication of adult spinal cord injury without radio graphic abnormality using magnetic resonance imaging: analysis of 40 patients. Surg Neurol. 2005; 63:204–209. PMID: 15734500.
10. Lim SH, Ko YJ, Shin JN, Kang SY, Moon SG, Kim JH. Functional recovery of patients with traumatic central cord syndrome. J Korean Acad Rehabil Med. 2002; 26:285–291.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download