1. Dossier C, Lapidus N, Bayer F, Sellier-Leclerc A, Boyer O, de Pontual L, et al. Epidemiology of idiopathic nephrotic syndrome in children: endemic or epidemic? Pediatr Nephrol. 2016; 31:2299–308.

2. Noone DG, Iijima K, Parekh R. Idiopathic nephrotic syndrome in children. The Lancet. 2018; 392:61–74.

3. Tullus K, Webb H, Bagga A. Management of steroid-resistant nephrotic syndrome in children and adolescents. The Lancet Child and Adolescent Health. 2018; 2:880–90.

4. Lombel RM, Gipson DS, Hodson EM. Treatment of steroid-sensitive nephrotic syndrome: New guidelines from KDIGO. Pediatric Nephrology. 2013; 28:415–26.

5. Canetta PA, Radhakrishnan J. The Evidence-Based Approach to Adult-Onset Idiopathic Nephrotic Syndrome. Front Pediatr. 2015; 3:78.

6. Stone H, Magella B, Bennett MR. The Search for biomarkers to aid in diagnosis, differentiation, and prognosis of childhood idiopathic nephrotic syndrome. Frontiers in Pediatrics. 2019; 7.

7. Trautmann A, Vivarelli M, Samuel S, Gipson D, Sinha A, Schaefer F, et al. IPNA clinical practice recommendations for the diagnosis and management of children with steroid-resistant nephrotic syndrome. Pediatric Nephrology. 2020; 35:1529–61.

8. Saleem MA. Molecular stratification of idiopathic nephrotic syndrome. Nat Rev Nephrol. 2019; 15:750–65.

9. Trautmann A, Schnaidt S, Lipska-Zietkiewicz BS, et al. Long-Term Outcome of Steroid-Resistant Nephrotic Syndrome in Children. J Am Soc Nephrol. 2017; 28:3055–65.
10. Lee JM, Kronbichler A, Shin JI, Oh J. Current understandings in treating children with steroid-resistant nephrotic syndrome. Pediatr Nephrol. 2021; 36:747–61.

11. Trautmann A, Ghiggeri GM, Azocar M, et al. Risk factors for posttransplant recurrence of steroid resistant nephrotic syndrome (SRNS): Results from the podonet registry. Pediatric Nephrology. 2015; 30:1557–8.
12. Kang HG, Ha IS, Cheong HI. Recurrence and Treatment after Renal Transplantation in Children with FSGS. Biomed Res Int. 2016; 2016:6832971.

13. Allen PJ, Chadban SJ, Craig JC, Lim WH, Allen RDM, Clayton PA, et al. Recurrent glomerulonephritis after kidney transplantation: risk factors and allograft outcomes. Kidney Int. 2017; 92:461–9.

14. Toshiyuki O, Hiroshi K, Motoshi H, Yasuhiro K, Yuko A, Michio N, Hiroshi S, et al. Effect of pre-and postoperative plasmapheresis on posttransplant recurrence of focal segmental glomerulosclerosis in children. Transplantation. 2001; 71:628–33.
15. McCarthy ET, Sharma M, Savin VJ. Circulating permeability factors in idiopathic nephrotic syndrome and focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2010; 5:2115–21.

16. Kopp JB, Anders HJ, Susztak K, Podestà MA, Friedhelm Hildebrandt GR, Romagnani P. Podocytopathies. Nat Rev Dis Primers. 2020; 6:68.

17. Bensimhon AR, Williams AE, Gbadegesin RA. Treatment of steroid-resistant nephrotic syndrome in the genomic era. Pediatric Nephrology. 2019; 34:2279–93.

18. Barnett HL, Edelmann CM Jr, Greifer I. The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. A report of the international study of kidney disease in children. Journal of Pediatrics. 1981; 98:561–4.
19. Abdelsalam SM. Prediction of steroid response in nephrotic syndrome by humoral immunity assessment. Journal of Clinical Immunology. 2012; 32:S270.
20. Asokan K, Malik A. The role of shear wave elastography in predicting the clinical outcome in paediatric patients with nephrotic syndrome. Pediatric Radiology. 2019; 49:S292–S3.
21. Azizov M, Umarova Z, Safarov Z, Akhmatalieva M, Valieva F. Hypoimmune conditions in children with nephrotic syndrome. Acta Paediatrica, International Journal of Paediatrics. 2010; 99:103.
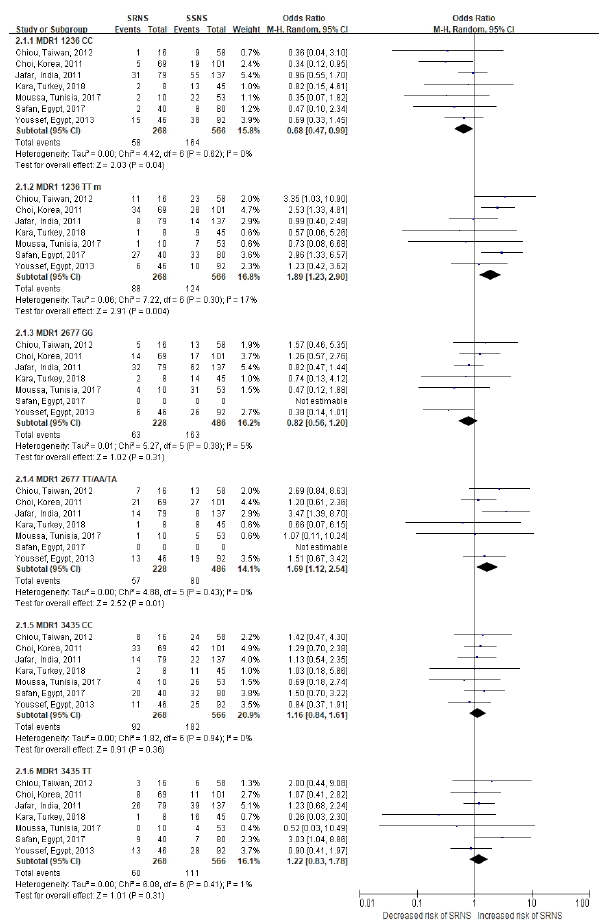
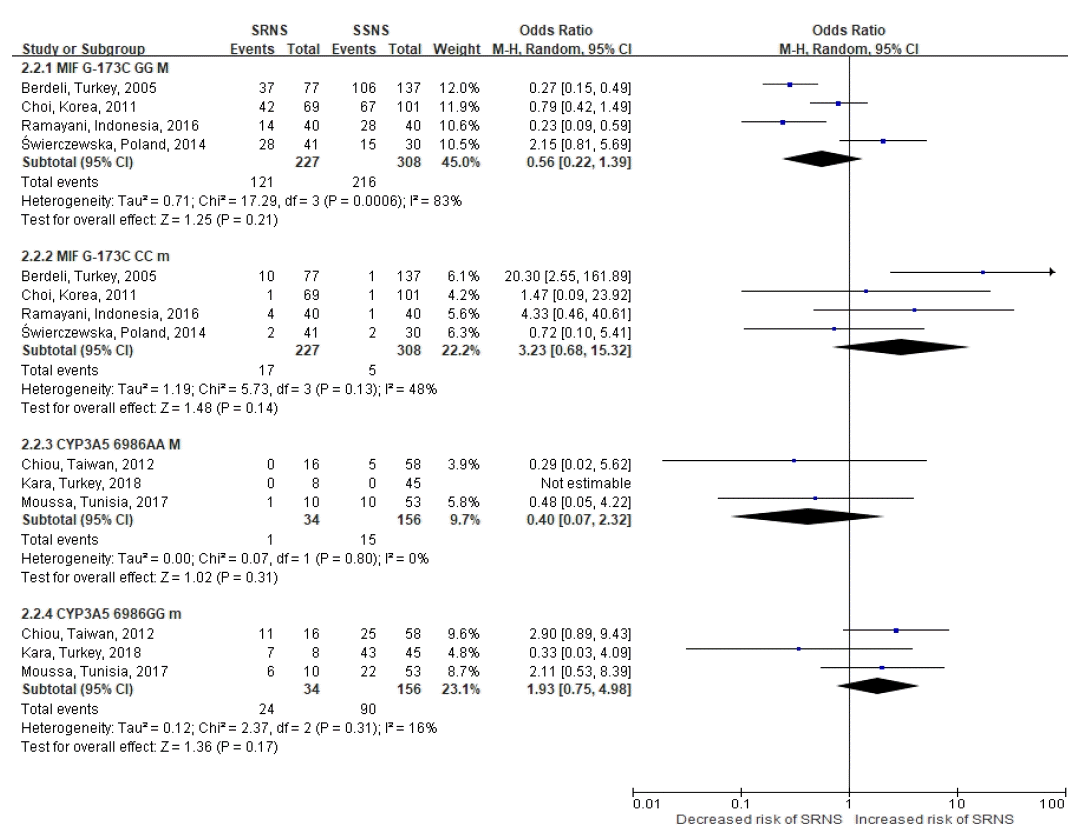
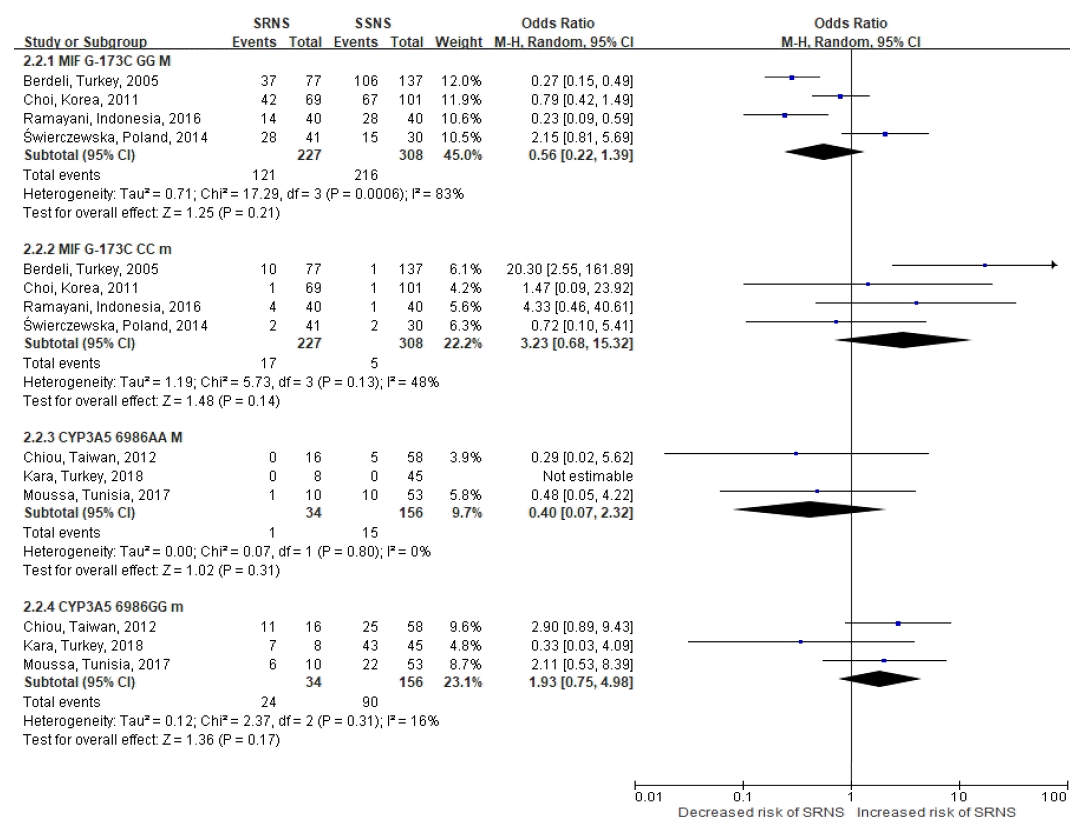
22. Chiou YH, Wang LY, Wang TH, Huang SP. Genetic polymorphisms of CYP3A5 and ABCB1 genes in steroid treatment of children with idiopathic nephrotic syndrome. Pediatric Nephrology. 2010; 25:1799–800.
23. Drannik GN, Driianska V, DuBuske LM. Assessment of HLA antigens and serum cytokine levels to predict disease progression and treatment responses in children with chronic glomerulonephritis. Journal of Allergy and Clinical Immunology. 2016; 137:AB114.

24. Garcia Martinez C, Bojórquez A. Mean platelet count as a prognostic biomarker in nephrotic syndrome. Blood Purification. 2018; 45:306.
25. Hussein AH, Ibrahim MAF, Ali MM, Mohamed ZMS. Evaluation of urinary annexin vas a prognostic marker in children with nephrotic syndrome. Nephrology Dialysis Transplantation. 2015; 30:iii147.
26. Jafar T, Prasad N, Mahdi AA. MDR-1 gene polymorphisms in steroid-responsive versus steroid-resistant nephrotic syndrome in children. Indian Journal of Clinical Biochemistry. 2017; 32:S76–S7.

27. Lipkowska K, Ostalska-Nowicka D, Smiech M, et al. The JAK/STAT signaling pathway modifications by glucocorticosteroids in the leukocytes of children with nephrotic syndrome. Nephrology Dialysis Transplantation. 2012; 27:ii324.
28. Liu T, Zhang B. Clinical significance of urine annexin a5 in primary nephrotic syndrome of children. Pediatric Nephrology. 2013; 28:1589.
29. Mishra OP, Kumar R, Narayan G, Srivastava P, Abhinay A, Prasad R, et al. Toll Like Receptor (TLR) -3, TLR-4 and CD 80 expressions in peripheral blood mononuclear cells and urinary CD 80 levels in children with idiopathic nephrotic syndrome. Pediatric Nephrology. 2016; 31:1828–9.
30. Prasad N, Jafar T, Agarwal V, Agarwal S, Sharma RK, Gupta A. MDR-1 gene polymorphisms in steroid responsive versus steroid resistant nephrotic syndrome in children. Nephrology. 2010; 15:99.
31. Prasad N, Jafar T, Agarwal V, Sharma RK, Agarwal S, Gupta A. Association of MDR-1 gene G2677T/A locus for TT/AA genotype with steroid resistance in children with nephrotic syndrome. NDT Plus. 2010; 3:iii278.
32. Sai S, Yamamoto M, Yamaguchi R, Chapman KE, Hongo T. Reciprocal regulation of 11ß-HSDS may predict steroid sensitivity in childhood nephrotic syndrome. Hormone Research in Paediatrics. 2017; 88:250.

33. Sharipov AM, Khamzayev KA. Immune condition in children with nephrotic syndrome. Intensive Care Medicine. 2012; 38:S183.
34. Silska M, Ostalska-Nowicka D, Smiech M, et al. SOCS1 over-expression in peripheral blood lymphocyte may predict resistance to steroids in childhood nephrotic syndrome. Pediatric Nephrology. 2010; 25:1883.
35. Singh H, Prasad N, Jaiswal AK, Agarwal V, Singh MK, Chauhan R. Expression and function of P-glycoprotein and multidrug resistanceassociated protein-1 and presence of homozygous mutant of multidrug resistance-associated protein-1 single nucleotide polymorphism G2677T/A identify steroid resistance phenotype in childhood idiopathic nephrotic syndrome. Indian Journal of Nephrology. 2019; 29:S30–S1.
36. Sinha A, Saini S, Saini H, Hari P, Bagga A. Urinary CD80 (uCD80), serum urokinase type plasminogen activator receptor (suPAR) and serum angiopoietin like 4 (Angptl4) do not distinguish steroid sensitive from steroid resistant nephrotic syndrome (NS). Pediatric Nephrology. 2016; 31:1839.
37. Weissbach A, Garty BZ, Krause I, Davidovits M. High serum TNF-alpha level is negatively correlated with steroid responsiveness in primary pediatric nephrotic syndrome. Pediatric Nephrology. 2013; 28:1583.
38. Youssef DM, Elbehidy RM, Abdelhalim HS, Amr GE. Soluble interleukine 2 receptor and MDR1 gene expression levels as inflammatory biomarkers for prediction of steroid response in children with nephrotic syndrome. Pediatric Nephrology. 2010; 25:1831.
39. Zurbig P, Ozaltin F, Anarat A, et al. Peptide biomarker signatures in steroidresistant nephrotic syndrome. Nephrology Dialysis Transplantation. 2018; 33:i630.
40. Yang J, Zhang BL. Value of determination of haptoglobin and α1- antitrypsin in predicting response to glucocorticoid therapy in children with primary nephrotic syndrome. Chinese Journal of Contemporary Pediatrics. 2015; 17:227–31.
41. Zhou TB, Qin YH, Su LN, Lei FY, Huang WF, Zhao YJ. ACE I/D gene polymorphism can't predict the steroid responsiveness in asian children with idiopathic nephrotic syndrome: A meta-analysis. PLoS ONE. 2011; 6.

42. Khurana M, Traum AZ, Aivado M, Wells MP, Guerrero M, Grall F, et al. Urine proteomic profiling of pediatric nephrotic syndrome. Pediatr Nephrol. 2006; 21:1257–65.

43. Calişkan S, Hacibekiroğlu M, Sever L, Ozbay G, Arisoy N. Urinary N-acetyl-beta-D-glucosaminidase and beta 2-microglobulin excretion in primary nephrotic children. Nephron. 1996; 74:401–4.
44. Mishra OP, Jain P, Srivastava P, Prasad R. Urinary N-acetyl-beta-D glucosaminidase (NAG) level in idiopathic nephrotic syndrome. Pediatr Nephrol. 2012; 27:589–96.

45. Piyaphanee N, Ma Q, Kremen O, Czech K, Greis K, Mitsnefes M, et al. Discovery and initial validation of α 1-B glycoprotein fragmentation as a differential urinary biomarker in pediatric steroidresistant nephrotic syndrome. Proteomics-Clinical Applications. 2011; 5:334–42.

46. Suresh CP, Saha A, Kaur M, Kumar R, Dubey NK, Basak T, et al. Differentially expressed urinary biomarkers in children with idiopathic nephrotic syndrome. Clin Exp Nephrol. 2016; 20:273–83.

47. Mastroianni Kirsztajn G, Nishida SK, Silva MS, Ajzen H, Pereira AB. Urinary retinol-binding protein as a prognostic marker in the treatment of nephrotic syndrome. Nephron. 2000; 86:109–14.

48. Simsek B, Buyukcelik M, Soran M, Bayazit AK, Noyan A, Seydaoglu G, et al. Urinary annexin V in children with nephrotic syndrome: A new prognostic marker? Pediatric Nephrology. 2008; 23:79–82.

49. Bennett MR, Piyaphanee N, Czech K, Mitsnefes M, Devarajan P. NGAL distinguishes steroid sensitivity in idiopathic nephrotic syndrome. Pediatric Nephrology. 2012; 27:807–12.

50. Nickavar A, Safaeian B, Sadeghi-Bojd S, Lahouti Harah dashti A. Urine Neutrophil Gelatinase Associated Lipocalin to Creatinine Ratio: A Novel Index for Steroid Response in Idiopathic Nephrotic Syndrome. Indian J Pediatr. 2016; 83:18–21.

51. Bennett MR, Pleasant L, Haffner C, Ma Q, Haffey WD, Ying J, et al. A novel biomarker panel to identify steroid resistance in childhood idiopathic nephrotic syndrome. Biomarker Insights. 2017; 12.

52. Lee H, Han KH, Lee SE, Kim SH, Kang HG, Cheong HI. Urinary exosomal WT1 in childhood nephrotic syndrome. Pediatr Nephrol. 2012; 27:317–20.

53. Mirkovic K, Doorenbos CR, Dam WA, Heerspink HJL, Slagman MCJ, Nauta FL, et al. Urinary vitamin D binding protein: a potential novel marker of renal interstitial inflammation and fibrosis. PLoS One. 2013; 8:e55887.

54. Bennett MR, Pordal A, Haffner C, Pleasant L, Ma Q, Devarajan P. Urinary vitamin D-binding protein as a biomarker of steroid-resistant nephrotic syndrome. Biomarker Insights. 2016; 11:1–6.

55. Cengiz N, Bayazit AK, Noyan A, Anarat R, Anarat A. Glycosaminoglycan excretion in children with nephrotic syndrome. Pediatric Nephrology. 2005; 20:486–90.

56. Gopal N, Koner BC, Bhattacharjee A, Bhat V. Assay of urinary protein-bound sialic acid can differentiate steroidsensitive nephrotic syndrome from steroid-resistant cases. Saudi J Kidney Dis Transpl. 2016; 27:37–40.

57. Shalhoub RJ. Pathogenesis of lipoid nephrosis: a disorder of T-cell function. Lancet. 1974; 2:556–60.

58. Mishra OP, Kumar R, Narayan G, Srivastava P, Abhinay A, Prasad R, et al. Toll-like receptor 3 (TLR-3), TLR-4 and CD80 expression in peripheral blood mononuclear cells and urinary CD80 levels in children with idiopathic nephrotic syndrome. Pediatric Nephrology. 2017; 32:1355–61.

59. Tain YL, Liu CA, Yang KD. Implications of blood soluble and cell surface tumor necrosis factor receptors in childhood nephrotic syndrome. Pediatric Nephrology. 2002; 17:926–32.

60. Guan FJ, Peng QQ, Wang LL, Yan XB, Dong C, Jiang XH. Histone deacetylase-2 expression and activity in children with nephrotic syndrome with different glucocorticoid response. Pediatr Nephrol. 2018; 33:269–76.

61. Youssef DM, Elbehidy RM, Abdelhalim HS, Amr GE. Soluble interleukine-2 receptor and MDR1 gene expression levels as inflammatory biomarkers for prediction of steroid response in children with nephrotic syndrome. Iran J Kidney Dis. 2011; 5:154–61.
62. Ling C, Wang X, Chen Z, Fan J, Meng Q, Zhou N, et al. Altered B-Lymphocyte homeostasis in Idiopathic Nephrotic Syndrome. Front Pediatr. 2019; 7:377.

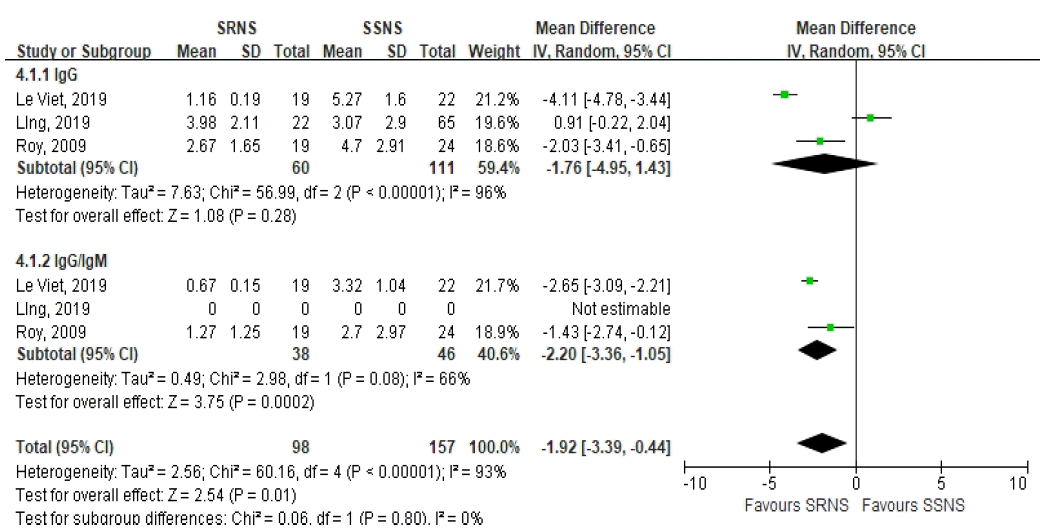
63. Roy RR, Roy E, Rahman MH, Hossain MM. Serum immunoglobulin G, M and IgG:IgM ratio as predictors for outcome of childhood nephrotic syndrome. World Journal of Pediatrics. 2009; 5:127–31.

64. Viet TL, Trung KN, Manh HD, Quy KT, Van TP, Van MC, et al. Serum igg level and igg/igm ratio on admission predict steroid-resistant response in vietnamese children with idiopathic nephrotic syndrome. Nephro-Urology Monthly. 2019; 11.

65. Wei C, Möller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, et al. Modification of kidney barrier function by the urokinase receptor. Nat Med. 2008; 14:55–63.

66. Sever S, Trachtman H, Wei C, Reiser J. Is there clinical value in measuring suPAR levels in FSGS? Clin J Am Soc Nephrol. 2013; 8:1273–5.

67. Maas RJ, Deegens JK, Wetzels JF. Serum suPAR in patients with FSGS: trash or treasure? Pediatr Nephrol. 2013; 28:1041–8.

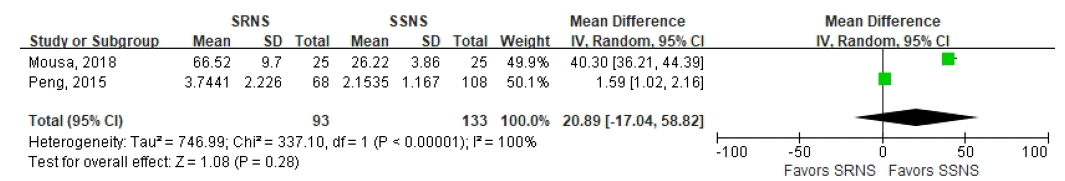
68. Peng Z, Mao J, Chen X, Cai F, Gu W, Fu H, et al. Serum suPAR levels help differentiate steroid resistance from steroid-sensitive nephrotic syndrome in children. Pediatr Nephrol. 2015; 30:301–7.

69. Mousa SO, Saleh SM, Aly HM, Amin MH. Evaluation of serum soluble urokinase plasminogen activator receptor as a marker for steroid-responsiveness in children with primary nephrotic syndrome. Saudi J Kidney Dis Transpl. 2018; 29:290–6.

70. Cuzzoni E, Franca R, De Iudicibus S, Marcuzzi A, Lucafò M, Pelin M, et al. MIF plasma level as a possible tool to predict steroid responsiveness in children with idiopathic nephrotic syndrome. European Journal of Clinical Pharmacology. 2019; 75:1675–83.
71. Watany MM, El-Horany HES. Nephronectin (NPNT) and the prediction of nephrotic syndrome response to steroid treatment. European Journal of Human Genetics. 2018; 26:1354–60.

72. Ahmed HM, Morgan DS, Doudar NA, Naguib MS. High Serum Endothelin-1 Level is Associated with Poor Response to Steroid Therapy in Childhood-Onset Nephrotic Syndrome. Saudi J Kidney Dis Transpl. 2019; 30:769–74.

73. Bakr A, Abul Hassan S, Shoker M, Zaki M, Hassan R. Oxidant stress in primary nephrotic syndrome: Does it modulate the response to corticosteroids? Pediatric Nephrology. 2009; 24:2375–80.

74. Ochocińska A, Jarmużek W, Janas R, Grenda R. Response to corticosteroid therapy is not related to serum and urine NGAL concentration in nephrotic children. Pediatria Polska. 2018; 93:245–50.

75. Fodor P, Saitúa MT, Rodriguez E, González B, Schlesinger L. T-cell dysfunction in minimal-change nephrotic syndrome of childhood. Am J Dis Child. 1982; 136:713–7.

76. Adalat S, Taylor J, Booth C, et al. Efficacy of rituximab in childhood nephrotic syndrome. Pediatric Nephrology. 2010; 25:1795.
77. Stachowski J, Barth C, Michałkiewicz J, Krynicki T, Jarmoliński T, Runowski D, et al. Th1/Th2 balance and CD45-positive T cell subsets in primary nephrotic syndrome. Pediatr Nephrol. 2000; 14:779–85.

78. Jaiswal A, Prasad N, Agarwal V, Yadav B, Tripathy D, Rai M, et al. Regulatory and effector T cells changes in remission and resistant state of childhood nephrotic syndrome. Indian J Nephrol. 2014; 24:349–55.

79. Nakajima H, Takenaka M, Kaimori JY, Hamano T, Iwatani H, Sugaya T, et al. Activation of the signal transducer and activator of transcription signaling pathway in renal proximal tubular cells by albumin. J Am Soc Nephrol. 2004; 15:276–85.

80. Ostalska-Nowicka D, Smiech M, Jaroniec M, Zaorska K, Zawierucha P, Szaflarski W, et al. SOCS3 and SOCS5 mRNA expressions may predict initial steroid response in nephrotic syndrome children. Folia Histochem Cytobiol. 2011; 49:719–28.

81. Zaorska K, Zawierucha P, Ostalska-Nowicka D, Nowicki M. SOCS3 is epigenetically up-regulated in steroid resistant nephrotic children. Acta biochimica Polonica. 2016; 63:131–8.

82. Hammad A, Yahia S, Gouida MS, Bakr A, El-farahaty RM. Low expression of glucocorticoid receptors in children with steroidresistant nephrotic syndrome. Pediatr Nephrol. 2013; 28:759–63.

83. Zahran AM, Aly SS, Elsayh KI, Badawy A, Gamal Y. Glucocorticoid receptors expression and histopathological types in children with nephrotic syndrome. Renal Failure. 2014; 36:1067–72.

84. Reiser J, von Gersdorff G, Loos M, Oh J, Asanuma K, Giardino L, et al. Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest. 2004; 113:1390–7.

85. Ji LD, Zhang LN, Shen P, Wang P, Zhang Y, Xing W, et al. Association of angiotensinogen gene M235T and angiotensin-converting enzyme gene I/D polymorphisms with essential hypertension in Han Chinese population: a meta-analysis. J Hypertens. 2010; 28:419–28.

86. Qin YH, Zhou TB, Su LN, Lei FY, Huang WF, Zhao YJ. Association between ACE polymorphism and risk of IgA nephropathy: a meta-analysis. J Renin Angiotensin Aldosterone Syst. 2011; 12:215–23.

87. Schena FP, D'Altri C, Cerullo G, Manno C, Gesualdo L. ACE gene polymorphism and IgA nephropathy: an ethnically homogeneous study and a meta-analysis. Kidney Int. 2001; 60:732–40.

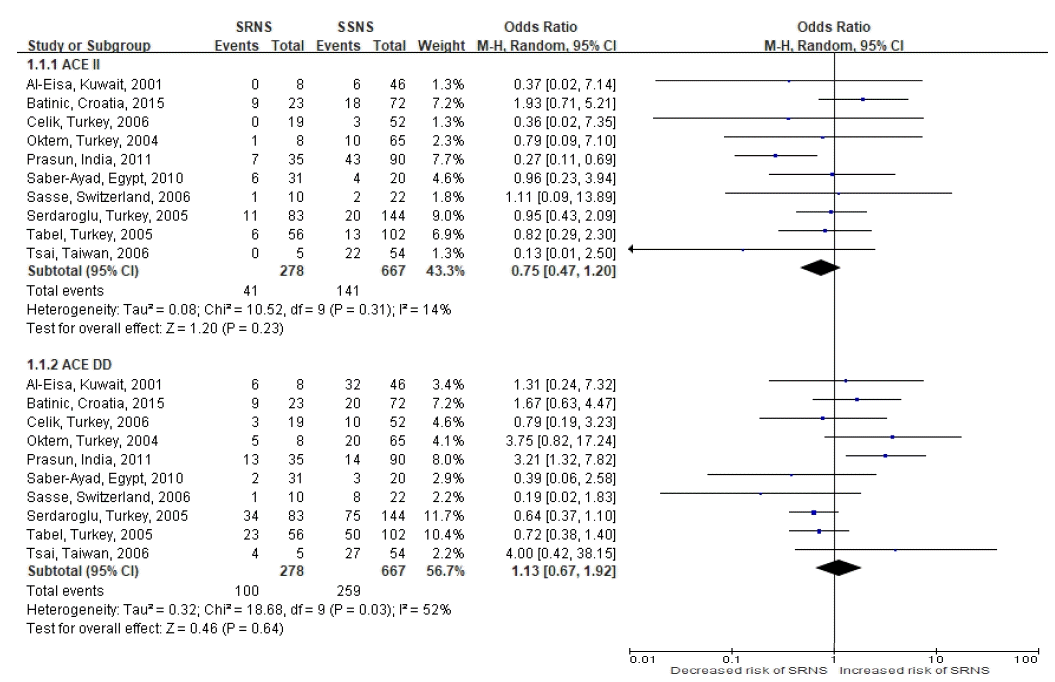
88. Al-Eisa A, Haider MZ, Srivastva BS. Angiotensin converting enzyme gene insertion/deletion polymorphism in idiopathic nephrotic syndrome in Kuwaiti Arab children. Scand J Urol Nephrol. 2001; 35:239–42.
89. Celik US, Noyan A, Bayazit AK, Büyükçelik M, Dursun H, Anarat A, et al. ACE gene polymorphism in Turkish children with nephrotic syndrome. Ren Fail. 2006; 28:401–3.
90. Prasun P, Prasad N, Tripathi G, Jafar T, Sharda S, Gulati S, et al. Association of angiotensin-converting enzyme gene I/D polymorphism with steroid responsiveness in childhood nephrotic syndrome. Indian J Nephrol. 2011; 21:26–9.

91. Sasse B, Hailemariam S, Wüthrich RP, Kemper MJ, Neuhaus TJ. Angiotensin converting enzyme gene polymorphisms do not predict the course of idiopathic nephrotic syndrome in Swiss children. Nephrology. 2006; 11:538–41.

92. Serdaroglu E, Mir S, Berdeli A, Aksu N, Bak M. ACE gene insertion/deletion polymorphism in childhood idiopathic nephrotic syndrome. Pediatr Nephrol. 2005; 20:1738–43.

93. Tsai IJ, Yang YH, Lin YH, Wu VC, Tsau YK, Hsieh FJ. Angiotensinconverting enzyme gene polymorphism in children with idiopathic nephrotic syndrome. Am J Nephrol. 2006; 26:157–62.

94. Batinic D, Sertic J, Coric M, Konjevoda P, Batinic D, Milosevic D. Angiotensin-converting enzyme genotype is not a significant genetic risk factor for idiopathic nephrotic syndrome in Croatian children. Nephron. 2015; 130:29–34.

95. Tabel Y, Berdeli A, Mir S, Serdaroglu E, Yilmaz E. Effects of genetic polymorphisms of the renin-angiotensin system in children with nephrotic syndrome. J Renin Angiotensin Aldosterone Syst. 2005; 6:138–44.

96. Abdel-Hafez M, Shimada M, Lee PY, Johnson RJ, Garin EH. Idiopathic Nephrotic Syndrome and Atopy: Is There a Common Link? American Journal of Kidney Diseases. 2009; 54:945–53.

97. Daehn I, Casalena G, Zhang T, Shi S, Fenninger F, Barasch N, et al. Endothelial mitochondrial oxidative stress determines podocyte depletion in segmental glomerulosclerosis. J Clin Invest. 2014; 124:1608–21.

98. Ezzat GM, Ali AB, Mohamed NA, Hetta HF. Association of endothelin receptor type A rs5333 gene polymorphism with steroid response in Egyptian children with idiopathic nephrotic syndrome. Pharmacogenomics. 2019; 20:133–41.

99. Attila G, Noyan A, Karabay Bayazit A, Acarturk E, Anarat A. Apolipoprotein E polymorphism in childhood nephrotic syndrome. Pediatr Nephrol. 2002; 17:359–62.

100. Ye J, Yu Z, Ding J, Chen Y, Huang J, Yao Y, et al. Genetic variations of the NR3C1 gene in children with sporadic nephrotic syndrome. Biochem Biophys Res Commun. 2006; 348:507–13.

101. Cheong HI, Kang HG, Schlondorff J. GLCCI1 single nucleotide polymorphisms in pediatric nephrotic syndrome. Pediatr Nephrol. 2012; 27:1595–9.
102. Choi HJ, Cho HY, Ro H, Lee SH, Han KH, Lee H, et al. Polymorphisms of the MDR1 and MIF genes in children with nephrotic syndrome. Pediatr Nephrol. 2011; 26:1981–8.

103. Jafar T, Prasad N, Agarwal V, et al. MDR-1 gene polymorphisms in steroid-responsive versus steroid-resistant nephrotic syndrome in children. Nephrol Dial Transplant. 2011; 26:3968–74.

104. Kara A, Gurgoze MK, Kara M, Aydin M. Evaluation of Genetic Polymorphisms for Determining Steroid Response in Nephrotic Children. Ann Clin Lab Sci. 2018; 48:478–83.
105. Moussa A, Mabrouk S, Hamdouni H, Ajmi M, Tfifha M, Omezzine A, et al. MDR-1 and CYP3A5 polymorphisms in pediatric idiopathic nephrotic syndrome: Impact on susceptibility and response to steroids (Preliminary Results). Clinical Laboratory. 2017; 63:1233–42.

106. Youssef DM, Attia TA, El-Shal AS, Abduelometty FA. Multi-drug resistance-1 gene polymorphisms in nephrotic syndrome: impact on susceptibility and response to steroids. Gene. 2013; 530:201–7.

107. Berdeli A, Mir S, Ozkayin N, Serdaroglu E, Tabel Y, Cura A. Association of macrophage migration inhibitory factor -173C allele polymorphism with steroid resistance in children with nephrotic syndrome. Pediatr Nephrol. 2005; 20:1566–71.

108. Vivarelli M, D'Urbano LE, Stringini G, et al. Association of the macrophage migration inhibitory factor -173*C allele with childhood nephrotic syndrome. Pediatr Nephrol. 2008; 23:743–8.

109. Ramayani OR, Sekarwana N, Trihono PP, Sadewa AH, Lelo A. A genetic study of steroid-resistant nephrotic syndrome: relationship between polymorphism -173 G to C in the MIF gene and serum level MIF in children. J Dev Orig Health Dis. 2016; 7:102–7.

110. Świerczewska M, Ostalska-Nowicka D, Kempisty B, Szczepankiewicz A, Nowicki M. Polymorphic variants of MIF gene and prognosis in steroid therapy in children with idiopathic nephrotic syndrome. Acta Biochim Pol. 2014; 61:67–75.

111. Chiou YH, Wang LY, Wang TH, Huang SP. Genetic polymorphisms influence the steroid treatment of children with idiopathic nephrotic syndrome. Pediatr Nephrol. 2012; 27:1511–7.

112. Turolo S, Edefonti A, Lepore M, Ghio L, Cuzzoni E, Decorti G, et al. SXR rs3842689: A prognostic factor for steroid sensitivity or resistance in pediatric idiopathic nephrotic syndrome. Pharmacogenomics. 2016; 17:1227–33.

113. Jafar T, Agrawal S, Mahdi AA, Sharma RK, Awasthi S, Agarwal GG. Cytokine gene polymorphism in idiopathic nephrotic syndrome children. Indian J Clin Biochem. 2011; 26:296–302.

114. Midan DAR, Elhelbawy NG, Habib MSE, Ahmedy IA, Noreldin RI. Cytokine Gene Polymorphism in Children With Idiopathic Nephrotic Syndrome. Iran J Kidney Dis. 2017; 11:414–21.
115. Youssef DM, El-Shal AS, Hussein S, Salah K, Ahmed A. Tumor necrosis factor alpha gene polymorphisms and haplotypes in Egyptian children with nephrotic syndrome. Cytokine. 2018; 102:76–82.

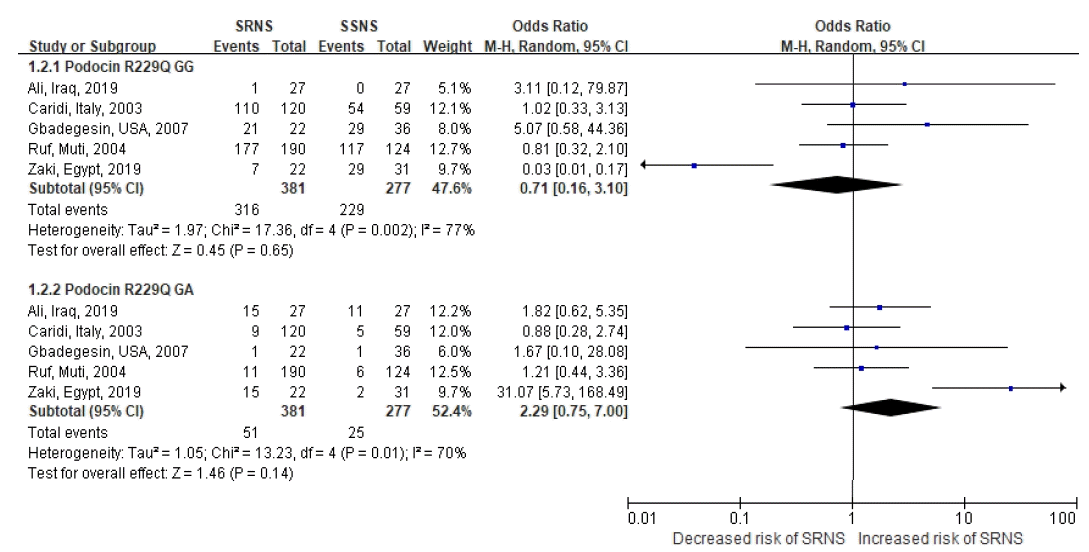
116. Caridi G, Bertelli R, Carrea A, Duca MD, Catarsi P, Artero M, et al. Prevalence, genetics, and clinical features of patients carrying podocin mutations in steroid-resistant nonfamilial focal segmental glomerulosclerosis. J Am Soc Nephrol. 2001; 12:2742–6.

117. Ruf RG, Lichtenberger A, Karle SM, Haas JP, Anacleto FE, Schultheiss M, et al. Patients with mutations in NPHS2 (podocin) do not respond to standard steroid treatment of nephrotic syndrome. J Am Soc Nephrol. 2004; 15:722–32.

118. Caridi G, Bertelli R, Di Duca M, Dagnino M, Emma F, Muda AO, et al. Broadening the spectrum of diseases related to podocin mutations. J Am Soc Nephrol. 2003; 14:1278–86.

119. Tsukaguchi H, Sudhakar A, Le TC, Nguyen T, Yao J, Schwimmer JA, et al. NPHS2 mutations in late-onset focal segmental glomerulosclerosis: R229Q is a common disease-associated allele. J Clin Invest. 2002; 110:1659–66.

120. Franceschini N, North KE, Kopp JB, McKenzie L, Winkler C. NPHS2 gene, nephrotic syndrome and focal segmental glomerulosclerosis: a HuGE review. Genet Med. 2006; 8:63–75.

121. Lu L, Wan H, Yin Y, Feng W, Wang M, Zou Y, et al. The p.R229Q variant of the NPHS2 (podocin) gene in focal segmental glomerulosclerosis and steroid-resistant nephrotic syndrome: a meta-analysis. Int Urol Nephrol. 2014; 46:1383–93.

122. Ali SH, Mohammed RK, Saheb HA, Abdulmajeed BA. R229Q Polymorphism of NPHS2 Gene in Group of Iraqi Children with Steroid-Resistant Nephrotic Syndrome. Int J Nephrol. 2017; 2017:1407506.

123. Gbadegesin R, Hinkes B, Vlangos C, Mucha B, Liu J, Hopcian J, et al. Mutational analysis of NPHS2 and WT1 in frequently relapsing and steroid-dependent nephrotic syndrome. Pediatr Nephrol. 2007; 22:509–13.

124. Zaki M, El-Shaer S, Rady S, El-Salam MA, Abd-El-Salam R, Alkashlan IA, et al. Analysis of NPHS2 Gene Mutations in Egyptian Children with Nephrotic Syndrome. Open Access Maced J Med Sci. 2019; 7:3145–8.

125. Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005; 308:1801–4.
126. Kuang XY, Huang WY, Xu H, Shi Y, Zhang X, Niu X, et al. 254C>G: a TRPC6 promoter variation associated with enhanced transcription and steroid-resistant nephrotic syndrome in Chinese children. Pediatr Res. 2013; 74:511–6.
127. Gulati S, Tripathi P, Patil SJ, Sharma RK, Agarwal S. Is typing for HLA class II alleles beneficial in Indian children with idiopathic nephrotic syndrome? Pediatric Nephrology. 2007; 22:528–32.

128. Ramanathan AS, Senguttuvan P, Chinniah R, Vijayan M, Thirunavukkarasu M, Raju K, et al. Association of HLA-DR/DQ alleles and haplotypes with nephrotic syndrome. Nephrology (Carlton). 2016; 21:745–52.

129. Schwarz R, Rossipal E. The prognosis of idiopathic nephrotic syndrome: a comparative study between the index of selectivity of proteinuria and the findings in renal biopsies. Padiatrie und Padologie. 1980; 15:131–6.
130. Lagrue G, Laurent J, Robeva R, Laurent G, Philippon C. Proteinuria selectivity index: prognostic value in idiopathic nephrotic syndromes. Annales de médecine interne. 1991; 142:249–53.
131. Zaki M, Deasy PF, Daoud AS. Proteinuria selectivity in childhood nephrotic syndrome. Bahrain Medical Bulletin. 1997; 19:15–7.
132. Choudhary A, Mohan Raj PS, Sonal S, Krishnamurthy S, Rajappa M. Association of urinary Vitamin-D binding protein and neutrophil gelatinase-associated lipocalin with steroid responsiveness in idiopathic nephrotic syndrome of childhood. Indian Journal of Clinical Biochemistry. 2018; 33:S90.
133. Kaneko K, Tuchiya K, Fujinaga S, Kawamura R, Ohtomo Y, Shimizu T, et al. Th1/Th2 balance in childhood idiopathic nephrotic syndrome. Clin Nephrol. 2002; 58:393–7.

134. Stachowski J, Barth C, Michalkiewicz J, Krynicki T, Jarmoliński T, Runowski D, et al. Th1/Th2 balance and CD45-positive T cell subsets in primary nephrotic syndrome. Pediatr Nephrol. 2000; 14:779–85.

135. Yap HK, Cheung W, Murugasu B, Sim SK, Seah CC, Jordan SC. Th1 and Th2 cytokine mRNA profiles in childhood nephrotic syndrome: evidence for increased IL-13 mRNA expression in relapse. J Am Soc Nephrol. 1999; 10:529–37.
136. Bustos C, Gonzalez E, Muley R, Alonso JL, Egido J. Increase of tumour necrosis factor alpha synthesis and gene expression in peripheral blood mononuclear cells of children with idiopathic nephrotic syndrome. Eur J Clin Invest. 1994; 24:799–805.
137. Agrawal S, Merchant ML, Kino J, Li M, Wilkey DW, Gaweda AE, et al. Predicting and Defining Steroid Resistance in Pediatric Nephrotic Syndrome Using Plasma Proteomics. Kidney International Reports. 2020; 5:66–80.

138. Gooding JR, Agrawal S, McRitchie S, Acuff Z, Merchant ML, Klein JB, et al. Predicting and Defining Steroid Resistance in Pediatric Nephrotic Syndrome Using Plasma Metabolomics. Kidney Int Rep. 2020; 5:81–93.

139. Han SS, Xu YQ, Lu Y, Gu XC, Wang Y. A PRISMA-compliant metaanalysis of MDR1 polymorphisms and idiopathic nephrotic syndrome: Susceptibility and steroid responsiveness. Medicine (Baltimore). 2017; 96:e7191.
140. Wang L, Li Q, Wang L, Li C, Yang H, Wang X, et al. The role of Th17/IL-17 in the pathogenesis of primary nephrotic syndrome in children. Kidney Blood Press Res. 2013; 37:332–45.

141. Kang HG, Seo H, Lim JH, Kim JI, Han KH, Park HW, et al. Markers of disease and steroid responsiveness in paediatric idiopathic nephrotic syndrome: Whole-transcriptome sequencing of peripheral blood mononuclear cells. J Int Med Res. 2017; 45:948–63.

142. Xing CY, Saleem MA, Coward RJ, Ni L, Witherden IR, Mathieson PW. Direct effects of dexamethasone on human podocytes. Kidney Int. 2006; 70:1038–45.

143. Zachwieja J, Bobkowski W, Dobrowolska-Zachwieja A, Lewandowska-Stachowiak M, Zaniew M, Maciejewski J. Intracellular cytokines of peripheral blood lymphocytes in nephrotic syndrome. Pediatr Nephrol. 2002; 17:733–40.
144. Zhou TB, Qin YH, Su LN, Lei F, Huang WF, Zhao Y, et al. Insertion/deletion (I/D) polymorphism of angiotensin-converting enzyme gene in steroid-resistant nephrotic syndrome for children: a genetic association study and meta-analysis. Ren Fail. 2011; 33:741–8.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download