Abstract
Since severe acute respiratory syndrome-coronavirus-2 variant B.1.1.529 (omicron) was first reported to the World Health Organization on November 24, 2021, the cases of the omicron variant have been detected in more than 90 countries over the last month. We investigated the clinical and epidemiological characteristics of the first 40 patients with the omicron variant who had been isolated at the National Medical Center in South Korea during December 4–17, 2021. The median age of the patients was 39.5 years. Twenty-two patients (55%) were women. Seventeen patients (42.5%) were fully vaccinated, and none were reinfected with the omicron. Eighteen (45%) had recent international travel history. Half of the patients (19, 47.5%) were asymptomatic, while the others had mild symptoms. Six patients (15%) showed lung infiltrations on chest image; however, none required supplemental oxygen. These mild clinical features are consistent with recent case reports on the omicron variant from other countries.
Graphical Abstract
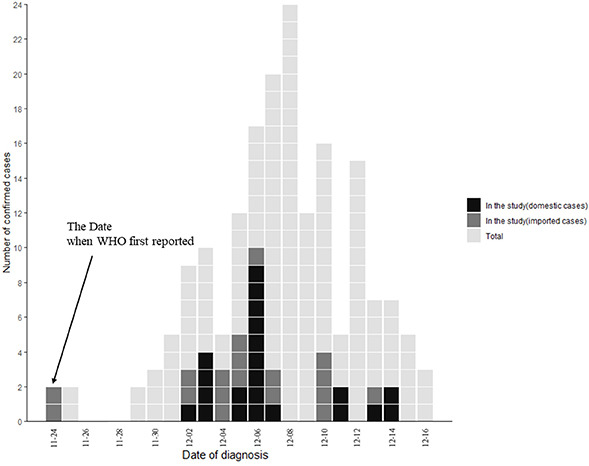
Severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) variant B.1.1.529 was first reported to the World Health Organization (WHO) from South Africa on November 24, 2021.1 Given its numerous mutations, particularly in the spike protein, there have been concerns about increased transmissibility, higher binding affinity, and escape from natural infection- or vaccine-induced immune responses.23 These potential concerns have shown a correlation with the recent steep increase in cases in South Africa.4 Therefore, the WHO designated SARS-CoV-2 variant B.1.1.529 as a variant of concern and named it omicron.1 Since the first identified case, the omicron variant has been detected in more than 90 countries, including South Korea, over the last month.5 The first cases (a married couple) in South Korea were reported on December 1, 2021;6 the couple had traveled to Nigeria and were confirmed to have been infected with this variant on November 25, 2021. Here, we investigated the clinical and epidemiological characteristics of the initial 40 patients infected with the omicron variant who had been isolated at our hospital.
Patients infected with the omicron variant have been isolated at the National Medical Center, a designated national hospital in South Korea. We included the initial 40 cases of the omicron variant infection during December 4–17, 2021. The omicron variant was confirmed by the Korea Disease Control and Prevention Agency with whole-genome sequencing. Demographic, epidemiological, and clinical characteristics of the patients were analyzed. Data on epidemiological characteristics including travel history, vaccination status, contact with confirmed patients, and date of last contact as well as information on clustered or sporadic cases were derived from history taking of patients and epidemiological investigation forms provided by public health centers. All patients underwent initial laboratory tests and radiologic examinations, including chest radiography and computed tomography (CT) on admission, except for children and a pregnant woman. After the initial evaluation, the changes in each patient’s symptoms and vital signs were monitored daily and recorded. Routine laboratory examinations and chest radiography were performed 7 days after admission and on the discharge date unless the patient’s condition had progressed.
The demographic, epidemiological, and clinical characteristics of the 40 omicron variant-confirmed patients are summarized in Tables 1 and 2. The median age was 39.5 years (interquartile range, 16–50.3; range, 2–69), and 11 patients (27.5%) were children aged < 18 years. Twenty-two patients (55%) were women. Seventeen patients (42.5%) were fully vaccinated: BNT162b2 (7, 41%), mRNA-1273 (5, 29%), ChAdOx1 (3, 18%), and Ad26.COV2.S (2, 12%). There were no known previously confirmed infection cases according to the patients’ statements. A total of 18 (45%) cases were considered imported; most were from South Africa (9, 22.5%) and Nigeria (6, 15%). Asymptomatic cases accounted for nearly half of the cases (19, 47.5%). All patients generally showed mild symptoms. The most common symptoms were sore throat (25%), fever (20%), headache (15%), cough (12.5%), and sputum production (12.5%). The mean duration of symptoms was 5.5 days (range, 2–11 days). Three patients needed antipyretics for managing persistent fever over 37.8 °C, which lasted 2–6 days. Based on chest radiography or CT findings on admission, six (15%) patients presented with lung infiltrations. Among them, three (50%) were aged > 50 years. None required supplemental oxygen therapy, antiviral agents, nor immunomodulatory agents for the treatment of coronavirus disease 2019 (COVID-19).
There is a previous epidemiological report on first 80 patients with SARS-CoV-2 omicron variant infection in South Korea.6 However, our study focused to investigate the clinical characteristics and hospital courses of the earliest 40 patients with omicron variant among them. The patients in our study were asymptomatic or showed mild symptoms that had resolved within several days; none needed supplemental oxygen, despite 10 persons (25%) aged over 50 being included in this cohort. These clinical features were consistent with recent findings from South Africa7 and other countries58 that the omicron variant might cause less severe disease than the Delta variant. Considering that our study included a small number of patients and most of them were young and without high-risk conditions more data are needed to determine the severity of the omicron variant.
In our cohort, half of the cases were infected via domestic transmission, even if majority were clustered cases. The US Centers for Disease Prevention and Control (CDC) recently announced that the omicron variant accounted for 73% of the COVID-19 cases in the US only 3 weeks after they reported that the first case was identified on December 1. The European CDC also reported that cases without an epidemiological link to travel are increasing and community-associated spread is occurring.5 Indeed, the last case of our study was imported from the US. This suggests that the effectiveness of travel restrictions for specific countries may be limited in the era of the worldwide spread of the omicron variant.
Our study has several limitations. We included only 40 patients in this urgent early report on the omicron variant. In addition, a small number of persons aged over 65 (n = 3, 7.5%) were included. Given that the older patients present with more severe COVID-19 disease, more data on the elderly group of patients are surely needed to evaluate the disease severity. Also, because more than half the cases were imported (n = 18; Korean 14, foreigner 4) in this study, more domestic cases are needed to define the epidemiologic and clinical features of omicron variant infection in South Korea.
The confirmed omicron variant infections have reached 227 cases in South Korea as of December 21, 2021, despite the strict ‘trace, test and treat’ strategy since the first reported case on December 1, 2021. We are facing a new tidal wave of SARS-CoV-2 infection, although we do not know whether it is more infectious, severe, or easily evades the immune response than other variants.9 Even though the severity of the disease is low, if the total number of confirmed cases surges, the absolute number of severe or critical cases could be substantial; this could worsen the shortage of medical resources. New evidence regarding omicron variants is rapidly accumulating worldwide but remains highly limited as of now. Further studies are needed to determine the nature of the new variants and their impacts on the current COVID-19 pandemic.
ACKNOWLEDGMENTS
We would like to thank the staff of isolation unit and Clinical Infectious Disease Research Center at the National Medical Center, South Korea.
Notes
Funding: This study was funded by the National Institute of Infectious Diseases, National Institute of Health, Korea Disease Control & Prevention Agency (2022-ER1601-00)
Author Contributions:
Conceptualization: Jeon J, Chung KH, Bang JH, Jang HC.
Methodology: Jeon J, Jang HC, Lee M.
Formal analysis: Jeon J, Kim MK, Lee KS, Sung HK.
Validation: Jeon J, Kim MK, Choi YY, Lee KS, Kim Y, Park JS, Um J, Lee M.
Investigation: Kim MK, Lee B, Choi YY, Kim Y.
Writing - original draft preparation: Jeon J, Kim MK, Lee B.
Writing - review & editing: Jeon J, Kim MK, Choi YY.
References
1. World Health Organization. Classification of omicron (B.1.1.529): SARS-CoV-2 variant of concern. Updated 2021. Accessed December 22, 2021.
https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern
.
2. Ingraham NE, Ingbar DH. The omicron variant of SARS-CoV-2: understanding the known and living with unknowns. Clin Transl Med. 2021; 11(12):e685. PMID: 34911167.

3. UK Health Security Agency. SARS-CoV-2 variants of concern and variants under investigation in England. Updated 2021. Accessed December 22, 2021.
https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1036501/Technical_Briefing_29_published_26_November_2021.pdf
.
4. Dyer O. Covid-19: South Africa’s surge in cases deepens alarm over omicron variant. BMJ. 2021; 375(3013):n3013. PMID: 34862184.

5. ECDC. Assessment of the further emergence and potential impact of the SARS-CoV-2 omicron variant of concern in the context of ongoing transmission of the Delta variant of concern in the EU/EEA, 18th. Updated 15 December 2021. Updated 2021. Accessed December 22, 2021.
https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-assessment-further-emergence-omicron-18th-risk-assessment-december-2021.pdf
.
6. Lee JJ, Choe YJ, Jeong H, Kim M, Kim S, Yoo H, et al. Importation and transmission of SARS-CoV-2 B.1.1.529 (omicron) variant of concern in Korea, November 2021. J Korean Med Sci. 2021; 36(50):e346. PMID: 34962117.

7. World Health Organization. Omicron spreads but severe cases remain low in South Africa. Updated 2021. Accessed December 22, 2021.
https://www.afro.who.int/news/omicron-spreads-severe-cases-remain-low-south-africa
.
8. CDC COVID-19 Response Team. SARS-CoV-2 B.1.1.529 (omicron) variant - United States, December 1–8, 2021. MMWR Morb Mortal Wkly Rep. 2021; 70(50):1731–1734. PMID: 34914670.
9. Karim SSA, Karim QA. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. 2021; 398(10317):2126–2128. PMID: 34871545.

Table 1
Demographics and baseline characteristics of 40 patients infected with the SARS-CoV-2 B.1.1.529 (omicron) variant
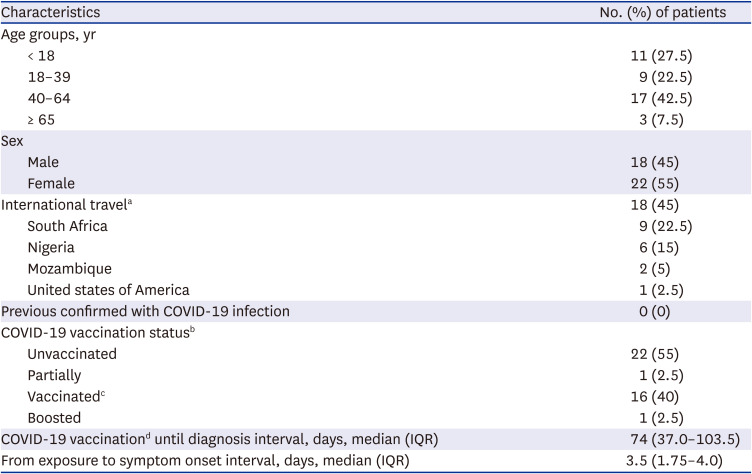
Data are shown as number (%) or median (IQRs).
SARS-CoV-2 = severe acute respiratory syndrome-coronavirus-2, COVID-19 = coronavirus disease 2019, IQR = interquartile range.
aInternational travel within 14 days before illness onset or receipt of a positive SARS-CoV-2 test result.
bUnvaccinated: a person who received no COVID-19 vaccination; Partially vaccinated: a person who received a COVID-19 vaccine dose but had not completed the approved primary series; Vaccinated: a person who completed an approved COVID-19 vaccine dose ≥ 14 days before illness onset or receipt of a positive SARS-CoV-2 test result; Boosted: a person who received an additional COVID-19 vaccine dose after completion of the approved primary series.
cFully vaccinated patients received BNT162b2 (7, 41%), mRNA-1273 (5, 29%), ChAdOx1 (3, 18%), and Ad26.COV2.S (2, 12%) vaccines.
dDay of completion of the primary schedule of COVID-19 vaccination.
Table 2
Clinical features and outcomes of 40 patients infected with the SARS-CoV-2 B.1.1.529 omicron variant
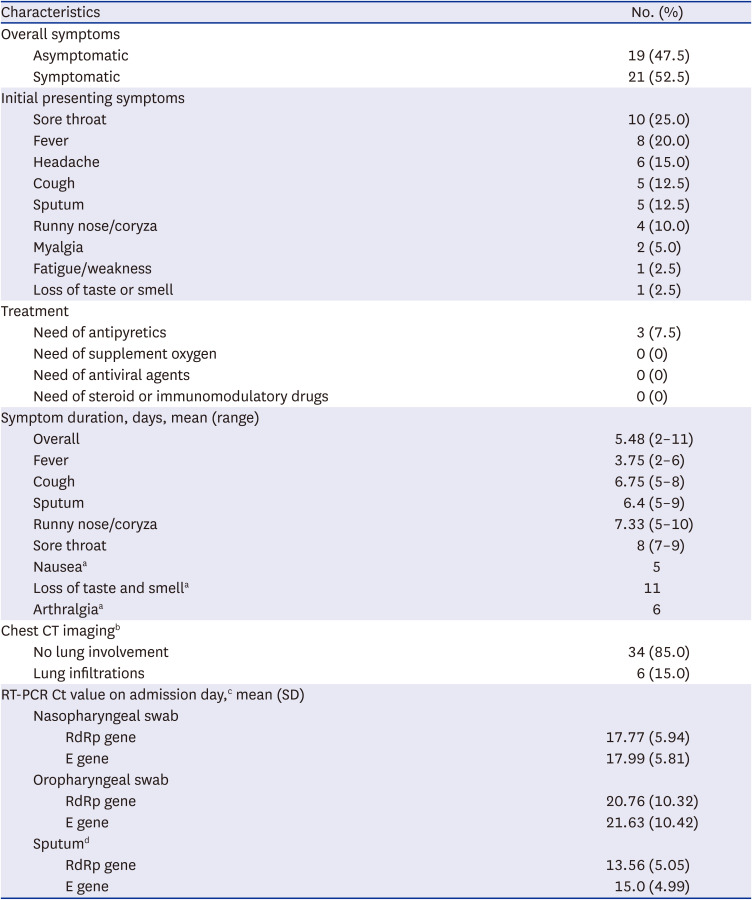
Data are shown as number (%) or mean (range) or mean (standard deviation).
SARS-CoV-2 = severe acute respiratory syndrome-coronavirus-2, CT = computed tomography, RT-PCR = reverse transcription polymerase chain reaction, Ct = cycle threshold, SD = standard deviation.
an = 1 for each item and range is unavailable.
bLow-dose chest CT images assessed for the presence of ground-glass opacities, consolidation, and ground-glass opacities with consolidation.
cn = 38 Person who could provide respiratory specimen for RT-PCR.
dn = 4 Persons who could provide specimen.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download