INTRODUCTION
Malignancy is an important cause of pericardial effusion (PE), ranging about 15–40% of significant PE.
1)2)3) In cancer patients with PE, pericardiocentesis is frequently required not only to relieve cardiac tamponade, but also to identify the etiology of PE. Although pericardiocentesis is a lifesaving, less invasive, and relatively safe procedure in cardiac tamponade, subsequent pericardial adhesions and constrictive physiology (CP) may follow, especially in cancer patients.
4)
In patients with cancer and PE, about half experience PE caused by malignant invasion,
5)6) thus PE is regarded as a poor prognostic indicator. Control of the underlying cancer is thought to be the only factor that influences survival.
7)8) However, the development of CP may be another significant prognostic indicator, since clinical symptoms such as dyspnea, edema, or arrhythmia persist even after pericardiocentesis, and may interrupt cancer therapy. However, there is a limited data on predicting survival and the likelihood of developing CP in patients with cancer and PE, especially after pericardiocentesis. We hypothesized that there would be differences in overall survival and characteristics between patients who develop CP and those who do not. Therefore, we investigated the medical history, laboratory, and imaging findings of cancer patients who underwent pericardiocentesis to identify risk factors that can predict the development of CP and the patients' survival.
Go to :

METHODS
Ethical statement
The study protocol was approved by the Institutional Review Board of Chonnam National University Hospital (IRB No. CNUHH-2020-190). A waiver for informed consent was obtained from the IRB. We have complied with the latest version of the Declaration of Helsinki (2013).
Study design and population
This is a multicenter retrospective study from 2 tertiary medical centers (Chonnam National University Hwasun Hospital and Chonnam National University Hospital, Republic of Korea). Pericardiocentesis was performed in 486 patients from January 2015 to January 2020. Among these, 156 were diagnosed with cancer within 5 years and being followed up by oncologists. After excluding 23 patients who did not have analyzable pre- or post-pericardiocentesis echocardiograms, a total of 133 were included in the analysis. The study population was divided into 2 groups: CP and non-CP.
Examinations and pericardiocentesis
Clinical information, including medical history and clinical symptoms, was obtained from each patient's medical record. Chest X-rays, 12-lead electrocardiograms (ECGs), serologic studies including complete blood counts, liver/renal function tests, and C-reactive protein (CRP), were performed for all patients. Chest computed tomography (CT) was performed in all patients on the day of or within 1 month of pericardiocentesis. Pericardial enhancement, thickening, or direct pericardial invasion was assessed by trained radiologists. Significant pericardial enhancement and thickening were defined as appearance of obviously contrast-enhanced parietal and visceral pericardial layers which can be distinguished from pericardial effusion. Echocardiography was performed at rest, in the left lateral decubitus position, by 2 trained sonographers using 2 digital echocardiographic equipment systems (Vivid E9 and E95; Vingmed Ultrasound, Horten, Norway), following the guidelines and diagnostic criteria of the American Society of Echocardiography and the European Association of Cardiovascular Imaging. In all cases, the left ventricular ejection fraction (LVEF) was measured using the modified biplane Simpson's method. The intraobserver and interobserver variabilities of Simpson's method were 4±5% and 5±4%, respectively. Other conventional measurements were performed in all cases. The size of PE was determined using a semiquantitative method to measure the depth of the pericardial space in diastole: scanty (not measurable), small (<10 mm), moderate (<20 mm), and large (>20 mm). Cardiac tamponade was defined by several echocardiographic criteria such as diastolic right atrial and right ventricular collapse, cardiac swinging motion, respiratory mitral inflow variation >25%, plethora of the inferior vena cava (IVC), and several clinical criteria such as arterial hypotension and/or tachycardia, which were relieved by pericardial fluid drainage and pulsus paradoxus.
9)
Image-guided (echocardiography and fluoroscopy) pericardiocentesis was performed by experienced cardiologists. A puncture needle was introduced into the pericardial space through the subxiphoid or intercostal route. An 8.5F-multihole pigtail catheter was introduced into the pericardial space for continuous drainage of pericardial fluid. The pericardial fluid underwent routine analysis (differential cell count, chemistry, microbiology, and pathology). After pericardiocentesis, echocardiography and chest radiography were performed to ensure correct catheter position and to identify acute mechanical complications (pericardial hematoma, pneumopericardium, pneumothorax, etc.). The catheter was retained until the drainage volume decreased to <50 mL for 24 hours. Follow-up echocardiography was then performed, and the catheter was removed if the residual PE volume was small (deepest pocket <10 mm).
Follow-up echocardiography was performed in the same way as pre-pericardiocentesis echocardiography. A diagnosis of CP was made when patients had features of elevated jugular venous pressure and at least one of the following: respirophasic ventricular septal shift, increase in early diastolic mitral inflow velocity (E velocity) >25% with expiration, medial mitral annular early diastolic velocity (medial e′ velocity) >8 cm/s and is higher than the lateral e′ velocity, prominent expiratory diastolic flow reversal in the hepatic veins, and IVC plethora.
10)
Statistical analyses
Statistical Package for the Social Sciences (SPSS) software (version 22.0; IBM Corp., Armonk, NY, USA) and R statistical software version 3.5.2. (R Foundation for Statistical Computing, Vienna, Austria) were used for all statistical analyses. All numerical variables are presented as mean value±standard deviation and were compared using an independent samples t-test or one-way analysis of variance. All categorical variables are shown as numbers and percentages, and they were compared using the χ
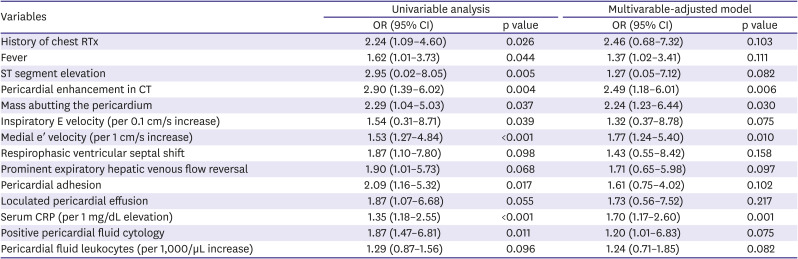
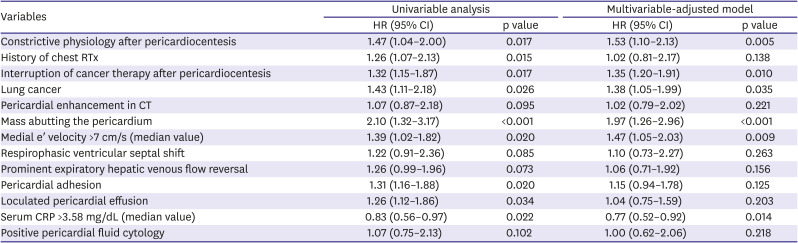
2 or Fisher's exact test to determine significance. To identify relation between CP and overall survival, Kaplan-Meier survival analysis was used. Logistic regression analysis was performed to determine the significant predictive factors of CP. Cox proportional hazard model was used to calculate the hazard ratio (HR) and the 95% confidence interval (CI) and to identify independent predictors of overall survival. Variables with p<0.15 in the univariable analysis were selected for the multivariable analysis. Interruption of cancer therapy was not considered for the logistic regression analysis, because it could not affect development of CP: interruption was determined in the oncologists' outpatient clinic after index hospitalization for pericardiocentesis. Significant multicollinearity between medial and lateral e′ velocity (variance inflation factor 16.536, 14.821, respectively) was detected. We chose medial e′ velocity over lateral e′ velocity, because medial e′ velocity is regarded as a more valuable echocardiographic parameter when determining constrictive physiology.
10) For Cox proportional hazard model, continuous variables were converted to dichotomous variables with the median value. All statistical tests were 2-tailed, and a p value <0.05 was considered significant.
Go to :

DISCUSSION
In this study, we investigated the imaging, laboratory, clinical characteristics and clinical outcomes of cancer patients who developed CP after pericardiocentesis in 2 large tertiary medical centers. We demonstrated the followings: First, CP commonly occurs after pericardiocentesis in cancer patients, regardless of the type of primary cancer. Second, there are differences in the characteristics of patients who developed CP after pericardiostomy and those who did not. These include pericardial enhancement on CT, the presence of a malignant mass abutting the pericardium, high serum CRP levels, and high medial e′ velocity. Third, CP is associated with significantly poor overall survival after pericardiocentesis, along with interruption of cancer therapy after pericardiocentesis, lung cancer, direct pericardial invasion of a malignant mass, high medial e′ velocity. Fourth, higher serum CRP levels was associated with better survival.
The incidence of CP after pericardiocentesis is reported to be much higher in cancer patients than in the general population.
10)11) It is even higher than the incidence of PE (approximately 1.3%) in pericarditis patients without cancer.
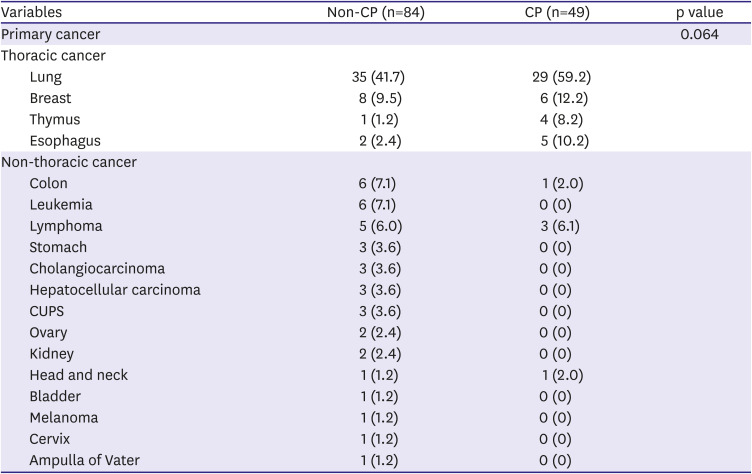
12) The higher incidence of CP in cancer patients can be partly attributed to recent advances in echocardiographic assessment and increased opportunities for the echocardiographic screening in cancer patients, but they cannot fully explain the extremely high incidence in this study (36.8%) and another recent large study (46.0%).
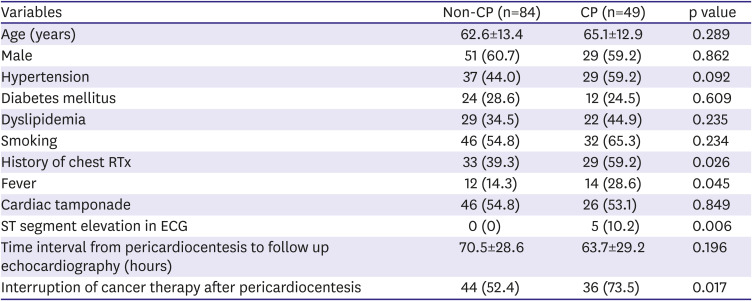
11) There may be different etiologies of CP in cancer patients than in the general population, such as direct malignant invasion, chest RTx, and adverse effects of some anti-cancer drugs.
13) This is reflected in our study by the high prevalence of malignant masses abutting the pericardium (35 patients, 26.3%).
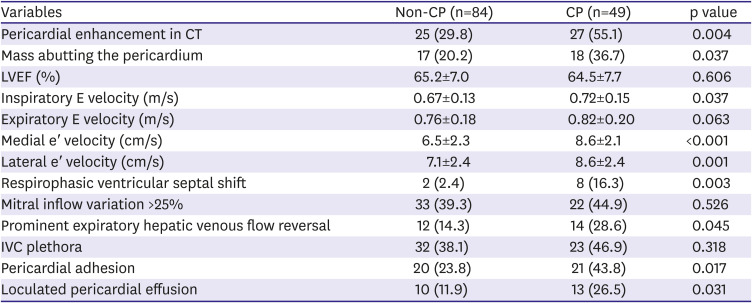
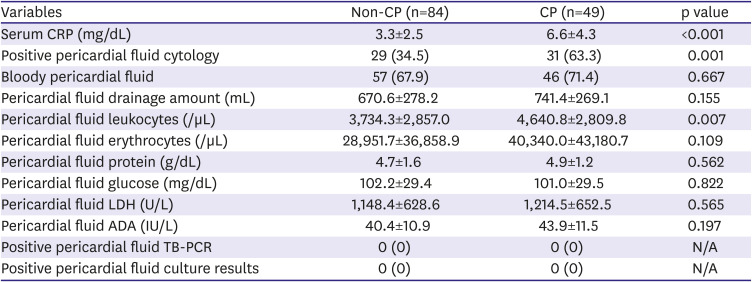
In this study, pericardial enhancement on CT was frequently observed in both groups, especially in the CP group. This is possibly due to malignant invasion, but in patients with pericardial enhancement, evidence of malignant invasion, such as abutting of a malignant mass or positive pericardial fluid cytology, was not universal. There may be different causes of pericardial diseases, other than cancer itself, in cancer patients. The presence of pericardial inflammation can be inferred with or without direct malignant invasion, due to other signs such as high serum CRP levels, high pericardial fluid leukocyte counts, fever, and ST segment elevation. Although pericardial trauma secondary to pericardiocentesis can cause pericardial inflammation, the inflammatory process is thought to begin even before pericardiocentesis because the signs described previously were detected before the procedure. Several important echocardiographic findings of CP, such as high medial e′ velocity, respirophasic ventricular septal shift, and prominent expiratory hepatic venous flow reversal,
14) were frequently observed on pre-pericardiocentesis echocardiography. Along with pericardial adhesions and consequent loculated PE, these are suggestive of preexisting pericardial inflammation even before pericardiocentesis.
Inflammation is thought to play a significant role in the development of effusive-constrictive pericarditis,
10)15) and it is a potential target of anti-inflammatory agents such as corticosteroids and colchicine to relieve or reverse pericardial adhesions and/or effusive-constrictive pericarditis in cancer patients after pericardiocentesis. Although lifesaving procedure, pericardiocentesis does not often improve the general condition and functional status of cancer patients. This may be due to cancer status; however, development of CP can also debilitate patients and interrupt further cancer therapy by causing dyspnea and intractable edema, or by limiting intravenous fluid therapy. CP itself may not be a more important cause of death than the cancer, but such heart failure by CP can be associated with poor overall survival in cancer patients after pericardiocentesis, as shown in our data. Although there are limited data on the efficacy of anti-inflammatory agents in cancer patients who develop CP, the development of CP risk assessment tools and anti-inflammatory treatment protocols could improve outcomes by enabling further cancer therapy and relieving heart failure symptoms.
The present study has some limitations. First, this was a retrospective study conducted in only 2 tertiary medical centers. Second, although the majority of included patients have lung or breast cancer, there are still many patients with other malignancies. Furthermore among patients with the same type of cancer, detailed histologic types, genotypes, and cancer therapies differed widely, which might have influenced the development of PE and CP. Third, we used comprehensive hemodynamic variables to define CP, but invasive cardiac catheterization was not performed in any patient; therefore, the true incidence of CP after pericardiocentesis may be different from our results. Fourth, although we performed comprehensive analysis of the pericardial fluid and the imaging findings in every patient, we could not fully distinguish between CP by malignancy and CP by other inflammatory causes. Fifth, since our data lacks long term follow up echocardiography results, CP status at longer term follow up could not be determined.
In conclusion, the present study suggests that CP frequently develops after pericardiocentesis, and it is associated with poor survival in cancer patients. In such patients, several clinical signs, imaging, and laboratory findings suggestive of pericardial inflammation and/or direct malignant pericardial invasion are frequently observed and could be used as predictors of CP development. Responsiveness to anti-inflammatory agents in cancer patients with CP according to these findings should be evaluated in further studies.
Go to :





 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download