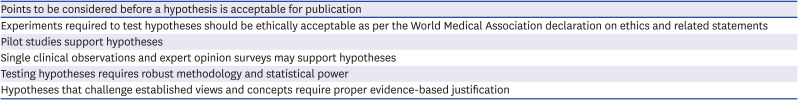
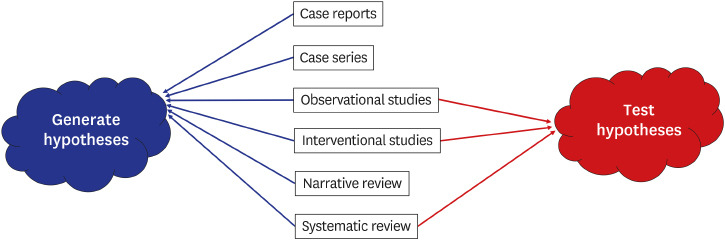
1. Gasparyan AY, Ayvazyan L, Mukanova U, Yessirkepov M, Kitas GD. Scientific hypotheses: writing, promoting, and predicting implications. J Korean Med Sci. 2019; 34(45):e300. PMID:
31760713.

2. Misra DP, Agarwal V. Generating working hypotheses for original research studies. Cent Asian J Med Hypotheses Ethics. 2020; 1(1):14–19.

3. Misra DP, Zimba O, Gasparyan AY. Statistical data presentation: a primer for rheumatology researchers. Rheumatol Int. 2021; 41(1):43–55. PMID:
33201265.

4. Yessirkepov M, Gasparyan AY. Embracing social media for generating and testing hypotheses. Cent Asian J Med Hypotheses Ethics. 2021; 2(3):133–136.

5. Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020; 382(10):929–936. PMID:
32004427.

6. Misra DP, Agarwal V. Systematic reviews: challenges for their justification, related comprehensive searches, and implications. J Korean Med Sci. 2018; 33(12):e92. PMID:
29542301.

7. Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD. Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int. 2011; 31(11):1409–1417. PMID:
21800117.

8. Röhrig B, du Prel JB, Wachtlin D, Blettner M. Types of study in medical research: part 3 of a series on evaluation of scientific publications. Dtsch Arztebl Int. 2009; 106(15):262–268. PMID:
19547627.
9. Misra DP, Agarwal V. Integrity of clinical research conduct, reporting, publishing, and post-publication promotion in rheumatology. Clin Rheumatol. 2020; 39(4):1049–1060. PMID:
32026178.

10. Smith GCS, Pell JP. Parachute use to prevent death and major trauma related to gravitational challenge: systematic review of randomised controlled trials. BMJ. 2003; 327(7429):1459–1461. PMID:
14684649.

11. Doll R, Hill AB. Smoking and carcinoma of the lung; preliminary report. BMJ. 1950; 2(4682):739–748. PMID:
14772469.
12. Goudsmit J. Alternative view on AIDS. Lancet. 1992; 339(8804):1289–1290. PMID:
1349680.

13. Duesberg P. Infectious AIDS--stretching the germ theory beyond its limits. Int Arch Allergy Immunol. 1994; 103(2):118–127. PMID:
8292899.
14. Dunlap RE, Jacques PJ. Climate change denial books and conservative think tanks: exploring the connection. Am Behav Sci. 2013; 57(6):699–731. PMID:
24098056.
15. Gupta L, Gasparyan AY, Misra DP, Agarwal V, Zimba O, Yessirkepov M. Information and misinformation on COVID-19: a cross-sectional survey study. J Korean Med Sci. 2020; 35(27):e256. PMID:
32657090.

16. Gupta L, Gasparyan AY, Zimba O, Misra DP. Scholarly publishing and journal targeting in the time of the coronavirus disease 2019 (COVID-19) pandemic: a cross-sectional survey of rheumatologists and other specialists. Rheumatol Int. 2020; 40(12):2023–2030. PMID:
33048199.

17. Parsonnet J. Clinician-discoverers--Marshall, Warren, and
H. pylori
. N Engl J Med. 2005; 353(23):2421–2423. PMID:
16339090.
18. Marshall B..
Helicobacter pylori--a Nobel pursuit? Can J Gastroenterol. 2008; 22(11):895–896. PMID:
19018331.
19. Riedel S. Edward Jenner and the history of smallpox and vaccination. Proc Bayl Univ Med Cent. 2005; 18(1):21–25. PMID:
16200144.

20. Heymann DL, Wilder-Smith A. Successful smallpox eradication: what can we learn to control COVID-19? J Travel Med. 2020; 27(4):taaa090. PMID:
32478398.

21. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020; 395(10229):1033–1034. PMID:
32192578.

22. Misra DP, Agarwal V, Gasparyan AY, Zimba O. Rheumatologists' perspective on coronavirus disease 19 (COVID-19) and potential therapeutic targets. Clin Rheumatol. 2020; 39(7):2055–2062. PMID:
32277367.

23. Misra DP, Gasparyan AY, Zimba O. Benefits and adverse effects of hydroxychloroquine, methotrexate and colchicine: searching for repurposable drug candidates. Rheumatol Int. 2020; 40(11):1741–1751. PMID:
32880032.

24. Shah S, Das S, Jain A, Misra DP, Negi VS. A systematic review of the prophylactic role of chloroquine and hydroxychloroquine in coronavirus disease-19 (COVID-19). Int J Rheum Dis. 2020; 23(5):613–619. PMID:
32281213.

25. Kim AHJ, Sparks JA, Liew JW, Putman MS, Berenbaum F, Duarte-García A, et al. A rush to judgment? Rapid reporting and dissemination of results and its consequences regarding the use of hydroxychloroquine for COVID-19. Ann Intern Med. 2020; 172(12):819–821. PMID:
32227189.

26. Sattui SE, Liew JW, Graef ER, Coler-Reilly A, Berenbaum F, Duarte-García A, et al. Swinging the pendulum: lessons learned from public discourse concerning hydroxychloroquine and COVID-19. Expert Rev Clin Immunol. 2020; 16(7):659–666. PMID:
32620062.

27. PRINCIPLE Trial Collaborative Group. Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet. 2021; 397(10279):1063–1074. PMID:
33676597.
28. Echeverría-Esnal D, Martin-Ontiyuelo C, Navarrete-Rouco ME, De-Antonio Cuscó M, Ferrández O, Horcajada JP, et al. Azithromycin in the treatment of COVID-19: a review. Expert Rev Anti Infect Ther. 2021; 19(2):147–163. PMID:
32853038.

29. Reardon S. Flawed ivermectin preprint highlights challenges of COVID drug studies. Nature. 2021; 596(7871):173–174. PMID:
34341573.

30. Butler-Laporte G, Nakanishi T, Mooser V, Morrison DR, Abdullah T, Adeleye O, et al. Vitamin D and COVID-19 susceptibility and severity in the COVID-19 host genetics initiative: a Mendelian randomization study. PLoS Med. 2021; 18(6):e1003605. PMID:
34061844.

31. The Lancet Diabetes Endocrinology. Vitamin D and COVID-19: why the controversy? Lancet Diabetes Endocrinol. 2021; 9(2):53. PMID:
33444566.
32. RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021; 384(8):693–704. PMID:
32678530.

33. Khan FA, Stewart I, Fabbri L, Moss S, Robinson K, Smyth AR, et al. Systematic review and meta-analysis of anakinra, sarilumab, siltuximab and tocilizumab for COVID-19. Thorax. 2021; 76(9):907–919. PMID:
33579777.

34. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021; 397(10285):1637–1645. PMID:
33933206.
35. Sauvat A, Ciccosanti F, Colavita F, Di Rienzo M, Castilletti C, Capobianchi MR, et al. On-target versus off-target effects of drugs inhibiting the replication of SARS-CoV-2. Cell Death Dis. 2020; 11(8):656. PMID:
32814759.

36. Tummino TA, Rezelj VV, Fischer B, Fischer A, O'Meara MJ, Monel B, et al. Drug-induced phospholipidosis confounds drug repurposing for SARS-CoV-2. Science. 2021; 373(6554):541–547. PMID:
34326236.

37. Escobar LE, Molina-Cruz A, Barillas-Mury C. BCG vaccine protection from severe coronavirus disease 2019 (COVID-19). Proc Natl Acad Sci U S A. 2020; 117(30):17720–17726. PMID:
32647056.

38. Gasparyan AY, Misra DP, Yessirkepov M, Zimba O. Perspectives of immune therapy in coronavirus disease 2019. J Korean Med Sci. 2020; 35(18):e176. PMID:
32383371.

39. Portnov BA, Dubnov J, Barchana M. On ecological fallacy, assessment errors stemming from misguided variable selection, and the effect of aggregation on the outcome of epidemiological study. J Expo Sci Environ Epidemiol. 2007; 17(1):106–121. PMID:
17033679.

40. Zimba O, Gasparyan AY. Peer review guidance: a primer for researchers. Reumatologia. 2021; 59(1):3–8. PMID:
33707789.
41. Yessirkepov M, Gasparyan AY. From testable hypotheses to ethical papers and improved health service. Cent Asian J Med Hypotheses Ethics. 2020; 1(1):10–13.

42. Wiseman R, Watt C, Kornbrot D. Registered reports: an early example and analysis. PeerJ. 2019; 7:e6232. PMID:
30671302.

43. Gaur PS, Zimba O, Agarwal V, Gupta L. Reporting survey based studies - a primer for authors. J Korean Med Sci. 2020; 35(45):e398. PMID:
33230988.

44. Zimba O, Radchenko O, Strilchuk L. Social media for research, education and practice in rheumatology. Rheumatol Int. 2020; 40(2):183–190. PMID:
31863133.

45. Ng W. Preprints as medium for communicating new ideas, hypotheses, data, analysis and beyond. PeerJ Prepr. 2017; 5:e3154v1.

46. Smith CM. Origin and uses of primum non nocere--above all, do no harm! J Clin Pharmacol. 2005; 45(4):371–377. PMID:
15778417.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download