Abstract
In 2020, the novel coronavirus disease 2019 (COVID-19) began to spread worldwide and remains an ongoing medical challenge. This case series reports on the clinical features and characteristics of patients with inflammatory bowel disease (IBD) and confirmed COVID-19 infection. From February 2020 to March 2021, nine patients with IBD had confirmed COVID-19 across four hospitals in Korea. The median age at COVID-19 diagnosis was 42 years. Six patients were male, and seven patients had ulcerative colitis (UC). No patients required oxygen therapy, intensive care unit hospitalizations, or died. The most common symptom was fever, and gastrointestinal (GI) symptoms developed as diarrhea in five patients with UC. Oral steroids were used to combat UC aggravation in two patients. In this case series of nine IBD patients diagnosed with COVID-19 in Korea, the clinical presentation was predominately a mild respiratory tract infection. Most patients with UC developed new GI symptoms including diarrhea.
Graphical Abstract
In 2020, the novel coronavirus disease 2019 (COVID-19) began to spread worldwide,1 and remains an ongoing medical challenge.2 The primary pathogenesis of COVID-19 is a respiratory infection, which can lead to severe pneumonia and acute respiratory distress syndrome, with a significantly higher mortality rate in patients with severe infection.3 Many cases of COVID-19 have occurred throughout Korea since the start of pandemic,4 but our understanding of the disease has progressed. Reports of patients with inflammatory bowel disease (IBD) confirmed with COVID-19 are increasing, and more is known about the impact of COVID-19 in these patients.56 Patients with IBD are reported to be at no greater risk of contracting COVID-19 than the general population.78 There have been no confirmed cases of IBD patients with confirmed COVID-19 in Korea yet. This case series reports on COVID-19 confirmed IBD patients' clinical features and characteristics in Korea.
This case series began with a survey of COVID-19 confirmed cases in patients with IBD at the IBD Study Group of the Korean Association for the Study of Intestinal Diseases. A surveillance questionnaire confirmed that nine patients with IBD in Korea had confirmed COVID-19 across four hospitals between February 2020 and March 2021. Based on this questionnaire, patients' symptoms and clinical courses were investigated at each institution. Patients who had confirmed COVID-19 and either Crohn's disease (CD) or ulcerative colitis (UC) were included in the study. A confirmed diagnosis of COVID-19 was defined as a positive result of reverse transcriptase-polymerase chain reaction (PCR) in a nasopharyngeal swab sample.9
We reviewed the medical records of eligible patients to investigate their baseline characteristics, IBD status, and IBD treatment. Patients' age, sex, IBD type, IBD disease activity before a diagnosis of COVID-19, IBD medication, and comorbid diseases were evaluated. Symptoms and clinical course of COVID-19 were investigated by reviewing the patients' medical records at the time of diagnosis or after full recovery. Respiratory symptoms, new gastrointestinal (GI) symptoms, laboratory and radiological findings, discontinuation of IBD medication, oxygen requirement, use of COVID-19 therapies, COVID-19 complications, and COVID-19 outcomes were also investigated. IBD disease activity was evaluated using the partial Mayo score (pMS) for patients with UC and the CD activity index for patients with CD.
In the surveillance questionnaire, nine patients with IBD (seven UC and two CD) and confirmed COVID-19 were identified. Their baseline characteristics, IBD type, and IBD treatment are shown in Table 1. Of them, 5 patients (55.6%) received 5-aminosalicylic acid (5-ASA) alone as a maintenance therapy. Two patients (22.2%) received biologics (infliximab for one patient with CD and vedolizumab for one patient with UC). The 5-ASA with an immunomodulator or corticosteroid was used to treat two patients. The patient on steroid treatment was diagnosed with COVID-19 while using prednisolone 10 mg during steroid tapering after steroid rescue therapy.
While infected COVID-19, 55.6% of patients experienced fever and 44.4% experienced myalgia. New GI symptoms emerged in 55.6% of patients, including diarrhea, abdominal pain, or bloody stools at the time of COVID-19 diagnosis. Diarrhea was identified as a new GI symptom in 5/7 patients with UC. Patients experienced COVID-19 symptoms for a mean during of 9.8 days. Six patients underwent testing for COVID-19 using PCR in the screening center and two in the hospital. Four patients were admitted to the hospital, and three were admitted to the residential treatment center. The remaining two patients were quarantined at home. Two patients had documented infection routes: one patient contracted the virus from a friend and the other from a family member.
One patient had focal patchy infiltration on chest radiography, and no patients required oxygen therapy, mechanical ventilation, or ICU admission. One patient was treated for COVID-19 with regdanvimab therapy. All patients could leave quarantine after the conditions for release had been met. All nine reported no complications related to COVID-19; however, one patient temporarily discontinued IBD medication after diagnosis of COVID-19. Azathioprine was temporarily halted in one patient using infliximab and azathioprine together because of decreased leukocyte count. Biologic therapy was used without discontinuation or postponement in two patients because the dosing interval did not overlap with the two-week COVID-19 treatment period. Two patients experienced IBD aggravation while infected with COVID-19. In one patient with UC under 5-ASA monotherapy with a pMS of 0, fecal calprotectin level was 66.8 µg/g at remission state. However, diarrhea and rectal bleeding started at COVID-19 diagnosis, and a pMS rose to 3. Symptoms persisted for 8 weeks after discharge with COVID-19 negative conversion and involved hospitalization; a pMS of 5 was confirmed. Symptoms improved after 8 weeks of oral beclomethasone administration and a pMS returned to 0. After 2 months, mild disease activity was observed with a pMS of 3, but the fecal calprotectin test was confirmed a level of 27,880 µg/g.
Another patient with ulcerative proctitis who used mesalamine suppository treatment was in remission before symptoms occurred. He had a pMS of 0; however, fecal calprotectin was high at 2,441 µg/g. Diarrhea and rectal bleeding occurred, and he was admitted to the hospital with a pMS of 6. Additionally, 10 days after several negative COVID-19 test, the patient tested positive. Despite focal patchy infiltration on chest radiography, oxygen therapy was not required, and the patient was initiated on regdanvimab therapy. After 24 days of management at a dedicated hospital, fever and respiratory symptoms improved, COVID-19 was negative converted, and patient was discharged. Despite continuous administration of oral 5-ASA during hospitalization, diarrhea and rectal bleeding did not subside for 36 days. The patient had a pMS of 8 and 4,758 µg/g fecal calprotectin level at the IBD clinic. After taking oral prednisolone 40 mg for 1-week, rectal bleeding improved, and the pMS improved to 2.
To the best of our knowledge, this study is the first case series in Asia to report on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in patients with IBD. Most of these patients diagnosed with COVID-19 in Korea were young and without comorbidities. There were no cases of severe COVID-19 that required intensive care or mechanical ventilation. Mild respiratory symptoms and diarrhea were characteristics of the infection with COVID-19 in the present study.
In April and May 2020, a number of case series on patients with IBD confirmed with COVID-19 emerged across Italy, Spain, and the United States.1011121314 Bezzio et al.10 reported that in 79 patients with IBD with COVID-19 in Italy, age > 65 years, prior comorbidities, and active IBD were associated with a greater severity of COVID-19. Also, Gubatan et al.12 reported five COVID-19 patients with IBD in the United States and reported that the positive rate of SARS-CoV-2 test in IBD patients was similar to that of the general population. Moreover, in a meta-analysis that reported the COVID-19 infection rate of 253,625 IBD patients, the COVID-19 infection rate of IBD patients was similar to that of the general population.15 In a matched cohort study in New York published by Lukin et al.,13 a comparison between COVID-19 patients with and without IBD showed that the proportion of severe COVID-19 did not differ. These findings match the results of our study in the Korean population. No patients in our study required intensive care nor mechanical ventilation; however, 88.9% of the patients in this series were under 50 years old, without comorbidities which were known as risk factors for severe course with COVID-19 infection.16
Previous studies have reported in detail the symptoms of COVID-19 patients with IBD. Taxonera et al.14 found that among 12 COVID-19 confirmed cases in a 1,918 IBD patient cohort in Spain, diarrhea was a characteristic symptom of infection in 75% of patients with IBD. Lukin et al.13 reported that diarrhea symptoms occurred more frequently in the IBD vs. non-IBD group (45% vs. 19%, respectively [P < 0.001]) and abdominal pain was also a more frequently reported GI manifestation in this patient population (20% vs. 5% [P = 0.001]). In a large meta-analysis of 25,252 patients in the general population diagnosed with COVID-19, 20.3% had GI manifestations, and 13.2% had diarrhea.17 Additionally, in a meta-analysis of COVID-19 symptoms in patients with IBD, 27.26% of 1,325 patients had diarrhea.18 The rate of diarrhea symptoms shown in both meta-analyses was higher in patients with IBD. Moreover, several studies have shown that COVID-19 patients with IBD had frequent GI symptoms, especially diarrhea, which occurred in 55.6% of patients in our study. The SARS-CoV-2 PCR test returned positive results from the feces of COVID-19 patients with GI symptoms, and intestinal epithelial cells were reportedly susceptible to SARS-CoV-2 infection.1920 In COVID-19 patients, GI symptoms may occur due to the effects of SARS-CoV-2. The effect of COVID-19 on IBD disease activity was not well known. In a recent study, Lukin et al.21 reported that it did not have a long-term effect of COVID-19 on IBD disorder activity. Therefore, further studies are needed on the GI effects of SARS-CoV-2 in patients with IBD. In this study, diarrhea and rectal bleeding continued in patients with IBD aggravation even after discharge from the hospital. However, these patients showed improvement in symptoms after oral steroid use. The COVID-19 pandemic has heavily impacted the methods for examining and managing patients with IBD. The Surveillance Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease (SECURE-IBD) study and multinational cohort study reported that systemic corticosteroid use increases the risk of severe COVID-19. Moreover, there was no evidence to suggest that use of 5-ASA, thiopurine or biologics in IBD patients with COVID-19 increases the risk of severe disease.222324 The International Organization for the Study of Inflammatory Bowel Diseases (IOIBD) and The European Crohn's and Colitis Organization (ECCO) made recommendations for managing patients with IBD diagnosed with COVID-19 based on expert opinion.2526 Adjusting medication according to COVID-19 severity and IBD disease activity was recommended. Patients with IBD were recommended to wash their hands, wear masks, keep social distance, and maintain medications for IBD during the COVID-19 pandemic. In patients with mild COVID-19 without hypoxia, symptom control, isolation, and continuing 5-ASA and topical rectal therapy were recommended. Additionally, discontinuing thiopurine, methotrexate, and tofacitinib and delaying biologics have been recommended. Systemic corticosteroids should be avoided and discontinued. New steroids should be cautiously introduced, and topical or oral budesonide are recommended.727 Furthermore, a non-endoscopy examination is preferred over an endoscopy for IBD patients with GI symptoms throughout the pandemic.28 Patients in our study did not undergo endoscopy and were followed up with symptom assessment, blood tests, and stool tests.
In summary, patients with IBD may develop new GI symptoms as the disease progresses. However, there are some limitations in treating and examining IBD patients during the COVID-19 pandemic, considering the frequent use of immunosuppressive agents in IBD.2930 Moreover, distinguishing between IBD aggravation and COVID-19 GI manifestation is challenging when new GI symptoms occur. During the COVID-19 pandemic, IBD patients with new GI symptoms such as diarrhea and abdominal pain should be screened with the SARS-CoV-2 test to discriminate against COVID-19-associated GI manifestations.
Notes
Funding: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) to Sang Hyoung Park (No.2021R1G1A1094252).
References
1. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395(10223):497–506. PMID: 31986264.

2. Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, evaluation, and treatment of coronavirus (COVID-19). StatPearls. Treasure Island, FL, USA: StatPearls Publishing;2021.
3. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020; 8(5):475–481. PMID: 32105632.

4. Seong H, Hyun HJ, Yun JG, Noh JY, Cheong HJ, Kim WJ, et al. Comparison of the second and third waves of the COVID-19 pandemic in South Korea: importance of early public health intervention. Int J Infect Dis. 2021; 104:742–745. PMID: 33556610.

5. Khan N, Mahmud N, Trivedi C, Reinisch W, Lewis JD. Risk factors for SARS-CoV-2 infection and course of COVID-19 disease in patients with IBD in the Veterans Affair Healthcare System. Gut. 2021; 70(9):1657–1664. PMID: 33753416.

6. Ludvigsson JF, Axelrad J, Halfvarson J, Khalili H, Larsson E, Lochhead P, et al. Inflammatory bowel disease and risk of severe COVID-19: a nationwide population-based cohort study in Sweden. United European Gastroenterol J. 2021; 9(2):177–192.

7. Kim KO, Jang BI. Management of inflammatory bowel disease in the COVID-19 era. Intest Res. Forthcoming. 2021; DOI: 10.5217/ir.2020.00156.

8. Maconi G, Bosetti C, De Monti A, Boyapati RK, Shelton E, Piazza N, et al. Risk of COVID 19 in patients with inflammatory bowel diseases compared to a control population. Dig Liver Dis. 2021; 53(3):263–270. PMID: 33483259.

9. Hong KH, Lee SW, Kim TS, Huh HJ, Lee J, Kim SY, et al. Guidelines for laboratory diagnosis of coronavirus disease 2019 (COVID-19) in Korea. Ann Lab Med. 2020; 40(5):351–360. PMID: 32237288.

10. Bezzio C, Saibeni S, Variola A, Allocca M, Massari A, Gerardi V, et al. Outcomes of COVID-19 in 79 patients with IBD in Italy: an IG-IBD study. Gut. 2020; 69(7):1213–1217. PMID: 32354990.

11. Allocca M, Fiorino G, Zallot C, Furfaro F, Gilardi D, Radice S, et al. Incidence and patterns of COVID-19 among inflammatory bowel disease patients from the Nancy and Milan Cohorts. Clin Gastroenterol Hepatol. 2020; 18(9):2134–2135. PMID: 32360811.

12. Gubatan J, Levitte S, Balabanis T, Patel A, Sharma A, Habtezion A. SARS-CoV-2 testing, prevalence, and predictors of COVID-19 in patients with inflammatory bowel disease in Northern California. Gastroenterology. 2020; 159(3):1141–1144.e2. PMID: 32387541.

13. Lukin DJ, Kumar A, Hajifathalian K, Sharaiha RZ, Scherl EJ, Longman RS. Baseline disease activity and steroid therapy stratify risk of covid-19 in patients with inflammatory bowel disease. Gastroenterology. 2020; 159(4):1541–1544.e2. PMID: 32479824.

14. Taxonera C, Sagastagoitia I, Alba C, Mañas N, Olivares D, Rey E. 2019 novel coronavirus disease (COVID-19) in patients with inflammatory bowel diseases. Aliment Pharmacol Ther. 2020; 52(2):276–283. PMID: 32359205.

15. Singh AK, Jena A, Kumar-M P, Sharma V, Sebastian S. Risk and outcomes of coronavirus disease in patients with inflammatory bowel disease: a systematic review and meta-analysis. United European Gastroenterol J. 2021; 9(2):159–176.

16. Sanyaolu A, Okorie C, Marinkovic A, Patidar R, Younis K, Desai P, et al. Comorbidity and its impact on patients with COVID-19. SN Compr Clin Med. 2020; 1–8.

17. Elshazli RM, Kline A, Elgaml A, Aboutaleb MH, Salim MM, Omar M, et al. Gastroenterology manifestations and COVID-19 outcomes: a meta-analysis of 25,252 cohorts among the first and second waves. J Med Virol. 2021; 93(5):2740–2768. PMID: 33527440.

18. Singh AK, Jena A, Kumar-M P, Jha DK, Sharma V. Clinical presentation of COVID-19 in patients with inflammatory bowel disease: a systematic review and meta-analysis. Intest Res. Forthcoming. 2021; DOI: 10.5217/ir.2020.00108.

19. Patrì A, Pinchera B, Spirito L, Delfino M, Imbimbo C, Salvatore P, et al. Gastrointestinal tract diseases as a risk factor for SARSCoV2 rectal shedding? An Italian report on 10 COVID-19 patients. Intest Res. 2021; 19(3):354–356. PMID: 33147898.

20. Barbosa da Luz B, de Oliveira NMT, França Dos Santos IW, Paza LZ, Braga LLVM, Platner FDS, et al. An overview of the gut side of the SARS-CoV-2 infection. Intest Res. 2021; 19(4):379–385. PMID: 33142370.

21. Lukin DJ, Funez-dePagnier G, Lima S, Lai D, Duenas-Bianchi L, Ahmed W, et al. No durable impact of COVID-19 on intestinal disease activity in subjects with IBD. Clin Gastroenterol Hepatol. 2021; 19(11):2312–2314.e3. PMID: 34102340.

22. Brenner EJ, Ungaro RC, Gearry RB, Kaplan GG, Kissous-Hunt M, Lewis JD, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020; 159(2):481–491.e3. PMID: 32425234.

23. Allocca M, Chaparro M, Gonzalez HA, Bosca-Watts MM, Palmela C, D'Amico F, et al. Patients with inflammatory bowel disease are not at increased risk of COVID-19: a large multinational cohort study. J Clin Med. 2020; 9(11):3533.

24. Attauabi M, Seidelin J, Burisch J. Danish COVID-IBD Study Group. Association between 5-aminosalicylates in patients with IBD and risk of severe COVID-19: an artefactual result of research methodology? Gut. 2021; 70(10):2020–2022. PMID: 33658323.

25. Allez M, Fleshner P, Gearry R, Lakatos PL, Rubin DT. Care of the patient with IBD requiring hospitalisation during the COVID-19 pandemic. J Crohns Colitis. 2020; 14(14 Suppl 3):S774–S779. PMID: 32722757.

26. Magro F, Rahier JF, Abreu C, MacMahon E, Hart A, van der Woude CJ, et al. Inflammatory bowel disease management during the COVID-19 outbreak: the ten do's and don'ts from the ECCO-COVID taskforce. J Crohns Colitis. 2020; 14(14 Suppl 3):S798–S806. PMID: 32722754.

27. Gajendran M, Perisetti A, Aziz M, Raghavapuram S, Bansal P, Tharian B, et al. Inflammatory bowel disease amid the COVID-19 pandemic: impact, management strategies, and lessons learned. Ann Gastroenterol. 2020; 33(6):591–602. PMID: 33162736.

28. Chen Y, Yu Q, Farraye FA, Kochhar GS, Bernstein CN, Navaneethan U, et al. Patterns of endoscopy during COVID-19 pandemic: a global survey of interventional inflammatory bowel disease practice. Intest Res. 2021; 19(3):332–340. PMID: 32475088.

29. Fukuda T, Naganuma M, Kanai T. Current new challenges in the management of ulcerative colitis. Intest Res. 2019; 17(1):36–44. PMID: 30678445.

30. Ooi CJ, Hilmi I, Banerjee R, Chuah SW, Ng SC, Wei SC, et al. Best practices on immunomodulators and biologic agents for ulcerative colitis and Crohn's disease in Asia. Intest Res. 2019; 17(3):285–310. PMID: 31146509.

Table 1
Clinical characteristics and outcomes in patients with IBD with COVID-19
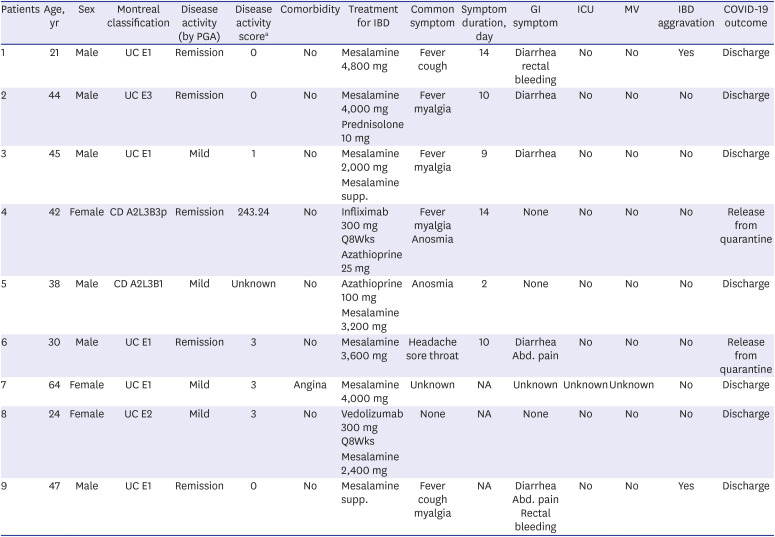
CD = Crohn's disease, IBD = inflammatory bowel disease, COVID-19 = coronavirus disease 2019, GI = gastrointestinal, ICU = intensive care unit, MV = mechanical ventilation, PGA = physician global assessment, UC = ulcerative colitis, NA = not available.
aDisease activity score presented partial Mayo score for UC patients and CD activity index for CD patients.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download