2. Aberegg SK, Cirulis MM, Maddock SD, Freeman A, Keenan LM, Pirozzi CS, et al. Clinical, bronchoscopic, and imaging findings of e-cigarette, or vaping, product use-associated lung injury among patients treated at an academic medical center. JAMA Netw Open. 2020; 3(11):e2019176. PMID:
33156346.

4. Chang YC, Lee YH, Liu CT, Shelley M. Patterns of e-cigarette use and self-reported health outcomes among smokers and non-smokers in the United States: a preliminary assessment. J Subst Use. 2019; 24(1):79–87.

7. Layden JE, Ghinai I, Pray I, Kimball A, Layer M, Tenforde MW, et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin - final report. N Engl J Med. 2020; 382(10):903–916. PMID:
31491072.

8. Jang SY, Cha Y, Yoo JI, Yu YT, Kim JT, Park CH, et al. Effect of pneumonia on all-cause mortality after elderly hip fracture: a Korean nationwide cohort study. J Korean Med Sci. 2020; 35(2):e9. PMID:
31920015.

10. Kang HS, Shin AY, Yeo CD, Kim JS, Kim YH, Kim JW, et al. A lower level of forced expiratory volume in one second predicts the poor prognosis of small cell lung cancer. J Thorac Dis. 2018; 10(4):2179–2185. PMID:
29850121.

11. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007; 370(9596):1453–1457. PMID:
18064739.

12. St Claire S, Gouda H, Schotte K, Fayokun R, Fu D, Varghese C, et al. Lung health, tobacco, and related products: gaps, challenges, new threats, and suggested research. Am J Physiol Lung Cell Mol Physiol. 2020; 318(5):L1004–L1007. PMID:
32233791.

13. Osei AD, Mirbolouk M, Orimoloye OA, Dzaye O, Uddin SM, Benjamin EJ, et al. Association between e-cigarette use and chronic obstructive pulmonary disease by smoking status: Behavioral Risk Factor Surveillance System 2016 and 2017. Am J Prev Med. 2020; 58(3):336–342. PMID:
31902685.

14. Xie W, Kathuria H, Galiatsatos P, Blaha MJ, Hamburg NM, Robertson RM, et al. Association of electronic cigarette use with incident respiratory conditions among US adults from 2013 to 2018. JAMA Netw Open. 2020; 3(11):e2020816. PMID:
33180127.

15. Chun LF, Moazed F, Calfee CS, Matthay MA, Gotts JE. Pulmonary toxicity of e-cigarettes. Am J Physiol Lung Cell Mol Physiol. 2017; 313(2):L193–L206. PMID:
28522559.

16. King BA, Jones CM, Baldwin GT, Briss PA. E-cigarette, or vaping, product use-associated lung injury: looking back, moving forward. Nicotine Tob Res. 2020; 22(Suppl 1):S96–S99. PMID:
33320257.

17. Casanova GS, Amaro R, Soler N, Sánchez M, Badía JR, Barberà JA, et al. An imported case of e-cigarette or vaping associated lung injury in Barcelona. Eur Respir J. 2020; 55(2):1902076. PMID:
31806720.

18. Blount BC, Karwowski MP, Shields PG, Morel-Espinosa M, Valentin-Blasini L, Gardner M, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020; 382(8):697–705. PMID:
31860793.

19. Ghosh A, Ahmad S, Coakley RD, Sassano MF, Alexis NE, Tarran R. Lipid-laden macrophages are not unique to patients with e-cigarette or vaping product use-associated lung injury. Am J Respir Crit Care Med. 2021; 203(8):1030–1033. PMID:
33332247.

20. Friedman AS. Association of vaping-related lung injuries with rates of e-cigarette and cannabis use across US states. Addiction. 2021; 116(3):651–657. PMID:
32840932.

21. Taylor J, Wiens T, Peterson J, Saravia S, Lunda M, Hanson K, et al. Characteristics of e-cigarette, or vaping, products used by patients with associated lung injury and products seized by law enforcement - Minnesota, 2018 and 2019. MMWR Morb Mortal Wkly Rep. 2019; 68(47):1096–1100. PMID:
31774740.

23. Boudi FB, Patel S, Boudi A, Chan C. Vitamin E acetate as a plausible cause of acute vaping-related illness. Cureus. 2019; 11(12):e6350. PMID:
31938636.

25. Filippidis FT, Laverty AA, Gerovasili V, Vardavas CI. Two-year trends and predictors of e-cigarette use in 27 European Union member states. Tob Control. 2017; 26(1):98–104. PMID:
27220621.

26. Bao W, Xu G, Lu J, Snetselaar LG, Wallace RB. Changes in electronic cigarette use among adults in the United States, 2014–2016. JAMA. 2018; 319(19):2039–2041. PMID:
29800201.

27. Jung JW, Kim JY. Characteristics of a “rebound” in smoking after a tobacco price increase. Int J Tuberc Lung Dis. 2020; 24(4):390–395. PMID:
32317062.

28. Kwon DS, Kim TH, Byun MK, Kim HJ, Lee HS, Park HJ, et al. Positive effects of the national cigarette price increase policy on smoking cessation in South Korea. Tuberc Respir Dis (Seoul). 2020; 83(1):71–80. PMID:
31905434.

29. Arnold MJ, Nollen NL, Mayo MS, Ahluwalia JS, Leavens EL, Zhang G, et al. Harm reduction associated with dual use of cigarettes and e-cigarettes in Black and Latino smokers: secondary analyses from a randomized controlled e-cigarette switching trial. Nicotine Tob Res. 2021; 23(11):1972–1976. PMID:
33837422.

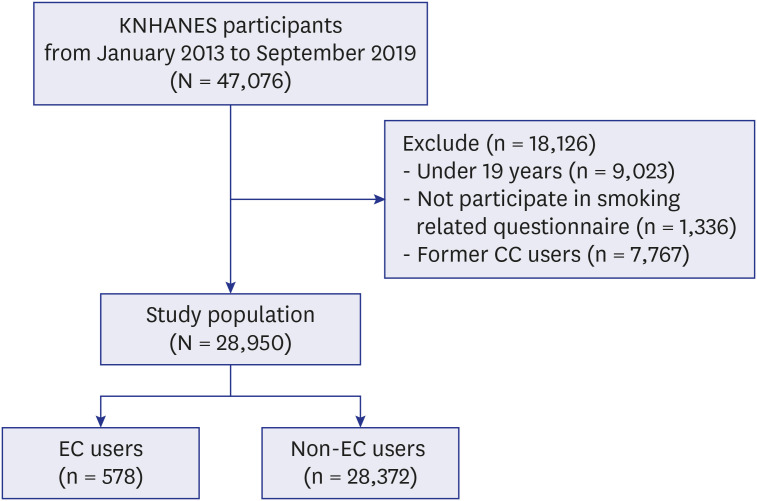
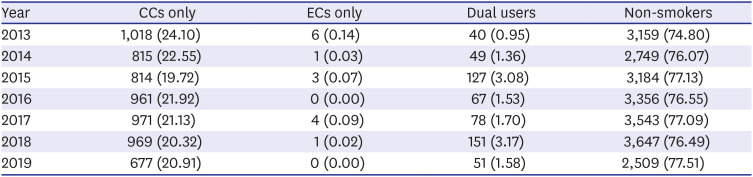
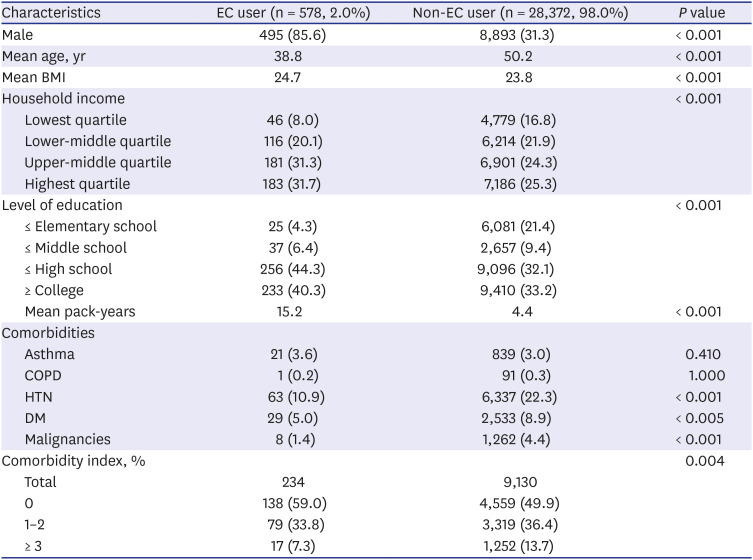
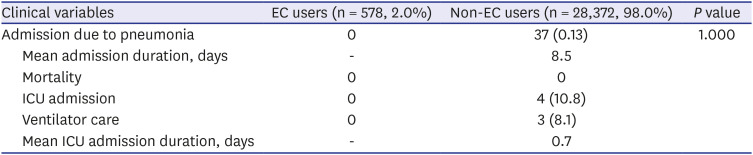




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download