2. Rahman MS, Ali I, Arooj M, Su XD, Yang SY, Kim YH, et al. Methyl 4-(β-D-glucopyranosyloxy)-3-hydroxy-5-methoxybenzoate, isolated from
Sanguisorba officinalis, inhibits CpG-DNA-induced inflammation.
Trop J Pharm Res 2020;19:1993-8.DOI:
10.4314/tjpr.v19i9.27.
3. Zhu F, Du B, Xu B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review.
Crit Rev Food Sci Nutr 2018;58:1260-70.DOI:
10.1080/10408398.2016.1251390. PMID:
28605204.
5. Yatoo MI, Gopalakrishnan A, Saxena A, Parray OR, Tufani NA, Chakraborty S, et al. Anti-inflammatory drugs and herbs with special emphasis on herbal medicines for countering inflammatory diseases and disorders-a review.
Recent Pat Inflamm Allergy Drug Discov 2018;12:39-58.DOI:
10.2174/1872213X12666180115153635. PMID:
29336271.
6. Nunes CDR, Barreto AM, Menezes de FPS, Leandro da CL, de Souza PM, Pereira de ML, et al. Plants as sources of anti-inflammatory agents.
Molecules 2020;25:3726.DOI:
10.3390/molecules25163726. PMID:
32824133. PMCID:
PMC7465135.
7. Bindu S, Mazumder S, Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective.
Biochem Pharmacol 2020;180:114147.DOI:
10.1016/j.bcp.2020.114147. PMID:
32653589. PMCID:
PMC7347500.
8. Oguntibeju OO. Medicinal plants with anti-inflammatory activities from selected countries and regions of Africa.
J Inflamm Res 2018;11:307-17.DOI:
10.2147/JIR.S167789. PMID:
30122972. PMCID:
PMC6086115.
10. Hou C, Chen L, Yang L, Ji X. An insight into anti-inflammatory effects of natural polysaccharides.
Int J Biol Macromol 2020;153:248-55.DOI:
10.1016/j.ijbiomac.2020.02.315. PMID:
32114173.
11. Tang C, Ding R, Sun J, Liu J, Kan J, Jin C. The impacts of natural polysaccharides on intestinal microbiota and immune responses- a review.
Food Funct 2019;10:2290-312.DOI:
10.1039/C8FO01946K. PMID:
31032827.
13. Xiong Q, Hao H, He L, Jing Y, Xu T, Chen J, et al. Anti-inflammatory and anti-angiogenic activities of a purified polysaccharide from flesh of
Cipangopaludina chinensis.
Carbohydr Polym 2017;176:152-9.DOI:
10.1016/j.carbpol.2017.08.073. PMID:
28927593.
14. Chen H, Sun J, Liu J, Gou Y, Zhang X, Wu X, et al. Structural characterization and anti-inflammatory activity of alkali-soluble polysaccharides from purple sweet potato.
Int J Biol Macromol 2019;131:484-94.DOI:
10.1016/j.ijbiomac.2019.03.126. PMID:
30904524.
15. Sun J, Gou Y, Liu J, Chen H, Kan J, Qian C, et al. Anti-inflammatory activity of a water-soluble polysaccharide from the roots of purple sweet potato.
RSC Adv 2020;10:39673-86.DOI:
10.1039/D0RA07551E.
16. Li S, Shah NP. Anti-inflammatory and anti-proliferative activities of natural and sulphonated polysaccharides from
Pleurotus eryngii.
J Funct Foods 2016;23:80-6.DOI:
10.1016/j.jff.2016.02.003.
17. Sanjeewa KKA, Fernando IPS, Kim EA, Ahn G, Jee Y, Jeon YJ. Anti-inflammatory activity of a sulfated polysaccharide isolated from an enzymatic digest of brown seaweed
Sargassum horneri in RAW 264.7 cells.
Nutr Res Pract 2017;11:3-10.DOI:
10.4162/nrp.2017.11.1.3. PMID:
28194259. PMCID:
PMC5300944.
18. Wang Y, Zhang N, Kan J, Zhang X, Wu X, Sun R, et al. Structural characterization of water soluble polysaccharide from
Arctium lappa and its effects on colitis mice.
Carbohydr Polym 2019;213:89-99.DOI:
10.1016/j.carbpol.2019.02.090. PMID:
30879693.
19. Zhang K, Liu Y, Zhao X, Tang Q, Dernedde J, Zhang J, et al. Anti-inflammatory properties of GLPss58, a sulfated polysaccharide from
Ganoderma lucidum.
Int J Biol Macromol 2018;107:486-93.DOI:
10.1016/j.ijbiomac.2017.09.015. PMID:
28890375.
20. Li Q, Li Q, Hao Z, Zheng X, He W. A novel polysaccharide from
Rhizoma panacis japonica exerts anti-inflammatory effects
via STAT3 signal pathway.
RSC Adv 2018;8:26371-6.DOI:
10.1039/C8RA02923G.
21. Rjeibi I, Feriani A, Saad AB, Ncib S, Sdayria J, Hfaiedh N, et al.
Lycium europaeum Linn as a source of polysaccharide with
in vitro antioxidant activities and
in vivo anti-inflammatory and hepato-nephroprotective potentials.
J Ethnopharmacol 2018;225:116-27.DOI:
10.1016/j.jep.2018.06.036. PMID:
29958959.
22. Lee SH, Ko CI, Jee Y, Jeong Y, Kim M, Kim JS, et al. Anti-inflammatory effect of fucoidan extracted from
Ecklonia cava in zebrafish model.
Carbohydr Polym 2013;92:84-9.DOI:
10.1016/j.carbpol.2012.09.066. PMID:
23218269.
23. Lee SH, Ko CI, Ahn G, You SG, Kim JS, Heu MS, et al. Molecular characteristics and anti-inflammatory activity of the fucoidan extracted from
Ecklonia cava. Carbohydr Polym 2012;89:599-606.DOI:
10.1016/j.carbpol.2012.03.056. PMID:
24750764.
24. Ren Y, Geng Y, Du Y, Li W, Lu ZM, Xu HY, et al. Polysaccharide of
Hericium erinaceus attenuates colitis in C57BL/6 mice via regulation of oxidative stress, inflammation-related signaling pathways and modulating the composition of the gut microbiota.
J Nutr Biochem 2018;57:67-76.DOI:
10.1016/j.jnutbio.2018.03.005. PMID:
29677563.
25. Wang Y, Zhu H, Wang X, Yu Y, Xie J. Natural food polysaccharides ameliorate inflammatory bowel disease and its mechanisms.
Foods 2021;10:1288.DOI:
10.3390/foods10061288. PMID:
34199820. PMCID:
PMC8227517.
26. Wang Y, Lai L, Teng L, Li Y, Cheng J, Chen J, et al. Mechanism of the anti-inflammatory activity by a polysaccharide from
Dictyophora indusiata in lipopolysaccharide-stimulated macrophages.
Int J Biol Macromol 2019;126:1158-66.DOI:
10.1016/j.ijbiomac.2019.01.022. PMID:
30625352.
27. Zhao R, Ji Y, Chen X, Su A, Ma G, Chen G, et al. Effects of a β-type glycosidic polysaccharide from
Flammulina velutipes on anti-inflammation and gut microbiota modulation in colitis mice.
Food Funct 2020;11:4259-74.DOI:
10.1039/C9FO03017D. PMID:
32356528.
28. Song W, Li Y, Zhang X, Wang Z. Potent anti-inflammatory activity of polysaccharides extracted from
Blidingia minima and their effect in a mouse model of inflammatory bowel disease.
J Funct Foods 2019;61:103494.DOI:
10.1016/j.jff.2019.103494.
29. Auyeung KK, Han QB, Ko JK. Astragalus membranaceus: a review of its protection against inflammation and gastrointestinal cancers.
Am J Chin Med 2016;44:1-22.DOI:
10.1142/S0192415X16500014. PMID:
26916911.
30. Wang K, Yang X, Wu Z, Wang H, Li Q, Mei H, et al.
Dendrobium officinale polysaccharide protected CCl
4-induced liver fibrosis through intestinal homeostasis and the LPS-TLR4-NF-κB signaling pathway.
Front Pharmacol 2020;11:240.DOI:
10.3389/fphar.2020.00240. PMID:
32226380. PMCID:
PMC7080991.
31. Liu Y, Lv J, Yang B, Liu F, Tian Z, Cai Y, et al.
Lycium barbarum polysaccharide attenuates type II collagen-induced arthritis in mice.
Int J Biol Macromol 2015;78:318-23.DOI:
10.1016/j.ijbiomac.2015.04.025. PMID:
25907010.
32. Chu X, Liu XJ, Qiu JM, Zeng XL, Bao HR, Shu J. Effects of
Astragalus and
Codonopsis pilosula polysaccharides on alveolar macrophage phagocytosis and inflammation in chronic obstructive pulmonary disease mice exposed to PM
2.5.
Environ Toxicol Pharmacol 2016;48:76-84.DOI:
10.1016/j.etap.2016.10.006. PMID:
27768989.
33. Javed S, Li WM, Zeb M, Yaqoob A, Tackaberry LE, Massicotte HB, et al. Anti-inflammatory activity of the wild mushroom,
Echinodontium tinctorium, in RAW264.7 macrophage cells and mouse microcirculation.
Molecules 2019;24:3509.DOI:
10.3390/molecules24193509. PMID:
31569655. PMCID:
PMC6804242.
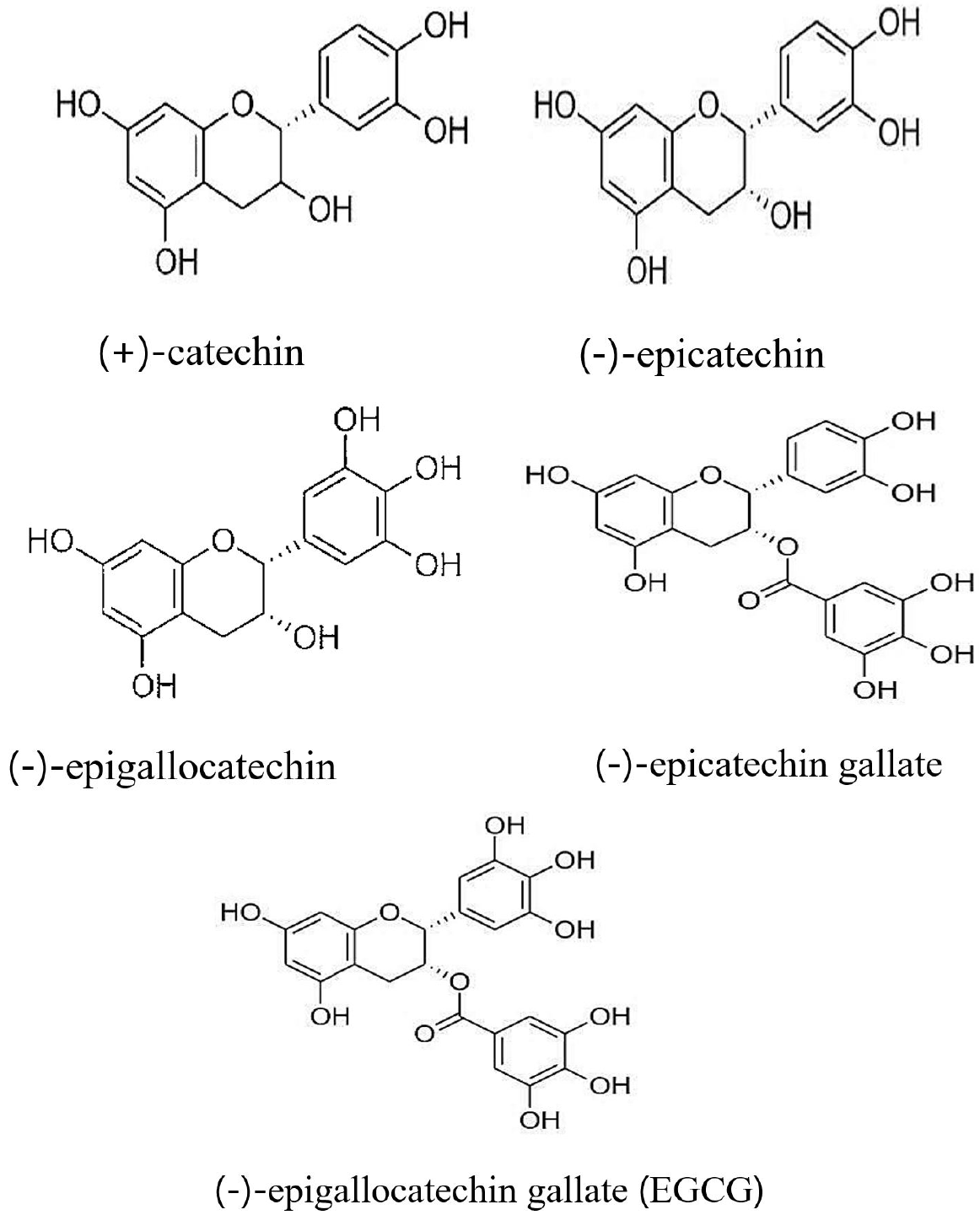
35. Reygaert WC. Green Tea Catechins: Their use in treating and preventing infectious diseases.
Biomed Res Int 2018;2018:9105261.DOI:
10.1155/2018/9105261. PMID:
30105263. PMCID:
PMC6076941.
37. Chatterjee P, Chandra S, Dey P, Bhattacharya S. Evaluation of anti-inflammatory effects of green tea and black tea: A comparative in vitro study.
J Adv Pharm Technol Res 2012;3:136-8.DOI:
10.4103/2231-4040.97298. PMID:
22837963. PMCID:
PMC3401676.
39. Tipoe GL, Leung TM, Liong EC, Lau TYH, Fung ML, Nanji AA. Epigallocatechin-3-gallate (EGCG) reduces liver inflammation, oxidative stress and fibrosis in carbon tetrachloride (CCl4)-induced liver injury in mice.
Toxicology 2010;273:45-52.DOI:
10.1016/j.tox.2010.04.014. PMID:
20438794.
40. Tang G, Xu Y, Zhang C, Wang N, Li H, Feng Y. Green Tea and Epigallocatechin Gallate (EGCG) for the Management of Nonalcoholic Fatty Liver Diseases (NAFLD): Insights into the Role of Oxidative Stress and Antioxidant Mechanism.
Antioxidants 2021;10:1076.DOI:
10.3390/antiox10071076. PMID:
34356308. PMCID:
PMC8301033.
41. Riegsecker S, Wiczynski D, Kaplan MJ, Ahmed S. Potential benefits of green tea polyphenol EGCG in the prevention and treatment of vascular inflammation in rheumatoid arthritis.
Life Sci 2013;93:307-12.DOI:
10.1016/j.lfs.2013.07.006. PMID:
23871988. PMCID:
PMC3768132.
43. Okabe S, Ochiai Y, Aida M, Park K, Kim SJ, Nomura T, et al. Mechanistic Aspects of Green Tea as a Cancer Preventive: Effect of Components on Human Stomach Cancer Cell Lines.
Jpn J Cancer Res 1999;90:733-9.DOI:
10.1111/j.1349-7006.1999.tb00808.x. PMID:
10470285. PMCID:
PMC5926138.
44. Yu Y, Deng Y, Lu BM, Liu YX, Li J, Bao JK. Green tea catechins: a fresh flavor to anticancer therapy.
Apoptosis 2014;19:1-18.DOI:
10.1007/s10495-013-0908-5. PMID:
24081390.
46. Adamczak A, Ożarowski M, Karpiński TM. Curcumin, a natural antimicrobial agent with strain-specific activity.
Pharmaceuticals 2020;13:153.DOI:
10.3390/ph13070153. PMID:
32708619. PMCID:
PMC7408453.
47. Kunnumakkara AB, Bordoloi D, Padmavathi G, Monisha J, Roy NK, Prasad S, et al. Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases.
Br J Pharmacol 2017;174:1325-48.DOI:
10.1111/bph.13621. PMID:
27638428. PMCID:
PMC5429333.
48. Koeberle A, Northoff H, Werz O. Curcumin blocks prostaglandin E2 biosynthesis through direct inhibition of the microsomal prostaglandin E2 synthase-1.
Mol Cancer Ther 2009;8:2348-55.DOI:
10.1158/1535-7163.MCT-09-0290. PMID:
19671757.
49. Khan Z, Khan N, Tiwari RP, Sah NK, Prasad GB, Bisen PS. Biology of Cox-2: an application in cancer therapeutics.
Curr Drug Targets 2011;12:1082-93.DOI:
10.2174/138945011795677764. PMID:
21443470.
54. Molfino A, Amabile MI, Monti M, Muscaritoli M. Omega-3 polyunsaturated fatty acids in critical illness: Anti- Inflammatory, Proresolving, or Both?
Oxid Med Cell Longev 2017;2017:5987082.DOI:
10.1155/2017/5987082. PMID:
28694914. PMCID:
PMC5488236.
56. Kostoglou-Athanassiou I, Athanassiou L, Athanassiou P. The effect of omega-3 fatty acids on rheumatoid arthritis.
Mediterr J Rheumatol 2020;31:190-4.DOI:
10.31138/mjr.31.2.190. PMID:
32676556. PMCID:
PMC7362115.
57. Gasparyan AY, Ayvazyan L, Yessirkepov M, Kitas GD. Colchicine as an anti-inflammatory and cardioprotective agent.
Expert Opin Drug Metab Toxicol 2015;11:1781-94.DOI:
10.1517/17425255.2015.1076391. PMID:
26239119.
61. Golpour M, Mousavi T, Alimohammadi M, Mosayebian A, Shiran M, Alizadeh Navaei R, et al. The effectiveness of Colchicine as an anti-inflammatory drug in the treatment of coronavirus disease 2019: Meta-analysis.
Int J Immunopathol Pharmacol 2021;35:20587384211031763.DOI:
10.1177/20587384211031763. PMID:
34250834. PMCID:
PMC8274088.
63. Abd Jalil MA, Kasmuri AR, Hadi H. Stingless bee honey, the natural wound healer: A review.
Skin Pharmacol Physiol 2017;30:66-75.DOI:
10.1159/000458416. PMID:
28291965.
64. Lim DCC, Bakar MFA, Majid M. Nutritional composition of stingless bee honey from different botanical origins.
IOP Conf Ser Earth Environ Sci 2019;269:012025. DOI:
10.1088/1755-1315/269/1/012025.
65. Shamsudin S, Selamat J, Sanny M, A R SB, Jambari NN, Khatib A. A Comparative characterization of physicochemical and antioxidants properties of processed
Heterotrigona itama honey from different origins and classification by chemometrics analysis.
Molecules 2019;24:3898.DOI:
10.3390/molecules24213898. PMID:
31671885. PMCID:
PMC6864699.
66. Ishii KJ, Koyama S, Nakagawa A, Coban C, Akira S. Host innate immune receptors and beyond: making sense of microbial infections.
Cell host microbe 2008;3:352-63.DOI:
10.1016/j.chom.2008.05.003. PMID:
18541212.
67. Trikha M, Corringham R, Klein B, Rossi JF. Targeted anti-interleukin-6 monoclonal antibody therapy for cancer: a review of the rationale and clinical evidence. Clin Cancer Res 2003;9:4653-65.
68. McInnes IB, Leung BP, Sturrock RD, Field M, Liew FY. Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-α production in rheumatoid arthritis.
Nat Med 1997;3:189-95.DOI:
10.1038/nm0297-189. PMID:
9018238.
69. Kawahara K, Hohjoh H, Inazumi T, Tsuchiya S, Sugimoto Y. Prostaglandin E2-induced inflammation: Relevance of prostaglandin E receptors.
Biochim Biophys Acta 2015;1851:414-21.DOI:
10.1016/j.bbalip.2014.07.008. PMID:
25038274.
70. Sharma JN, Al-Omran A, Parvathy SS. Role of nitric oxide in inflammatory diseases.
Inflammopharmacology 2007;15:252-9.DOI:
10.1007/s10787-007-0013-x. PMID:
18236016.
71. Kim CS, Kawada T, Kim BS, Han IS, Choe SY, Kurata T, et al. capsaicin exhibits anti-inflammatory property by inhibiting IκBα degradation in LPS-stimulated peritoneal macrophages.
Cell Signal 2003;15:299-306.DOI:
10.1016/S0898-6568(02)00086-4.
72. Fattori V, Hohmann MS, Rossaneis AC, Pinho-Ribeiro FA, Verri WA. Capsaicin: current understanding of its mechanisms and therapy of pain and other pre-clinical and clinical uses.
Molecules 2016;21:844.DOI:
10.3390/molecules21070844. PMID:
27367653. PMCID:
PMC6273101.
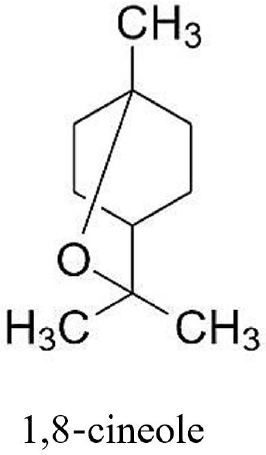
75. Juergens UR. Anti-inflammatory properties of the monoterpene 1.8-cineole: current evidence for co-medication in inflammatory airway diseases.
Drug Res 2014;64:638-46.DOI:
10.1055/s-0034-1372609. PMID:
24831245.
76. Juergens LJ, Worth H, Juergens UR. New perspectives for mucolytic, anti-inflammatory and adjunctive therapy with 1,8-cineole in COPD and asthma: review on the new therapeutic approach.
Adv Ther 2020;37:1737-53.DOI:
10.1007/s12325-020-01279-0. PMID:
32200535. PMCID:
PMC7467491.
78. Imran M, Rauf A, Shah ZA, Saeed F, Imran A, Arshad MU, et al. Chemo‐preventive and therapeutic effect of the dietary flavonoid kaempferol: A comprehensive review.
Phytother Res 2019;33:263-75.DOI:
10.1002/ptr.6227. PMID:
30402931.
79. Calderon-Montano JM, Burgos-Morón E, Pérez-Guerrero C, López-Lázaro M. A review on the dietary flavonoid kaempferol.
Mini Rev Med Chem 2011;11:298-344.DOI:
10.2174/138955711795305335. PMID:
21428901.
80. Cocate PG, Kac G, Heitmann BL, Nadanovsky P, da Veiga Soares Carvalho MC, Benaim C, et al. Calcium and vitamin D supplementation and/or periodontal therapy in the treatment of periodontitis among Brazilian pregnant women: protocol of a feasibility randomized controlled trial (the IMPROVE trial).
Pilot Feasibility Stud 2019;5:38.DOI:
10.1186/s40814-019-0417-6. PMID:
30873290. PMCID:
PMC6402123.
81. Owczarek D, Rodacki T, Domagała-Rodacka R, Cibor D, Mach T. Diet and nutritional factors in inflammatory bowel diseases.
World J Gastroenterol 2016;22:895-905.DOI:
10.3748/wjg.v22.i3.895. PMID:
26811635. PMCID:
PMC4716043.
83. Barbalho SM, Goulart RdA, Batista GLDSA. Vitamin A and inflammatory bowel diseases: from cellular studies and animal models to human disease.
Expert Rev Gastroenterol Hepatol 2019;13:25-35.DOI:
10.1080/17474124.2019.1543588. PMID:
30791845.
84. Bessler H, Wyshelesky G, Osovsky M, Prober V, Sirota L. A comparison of the effect of vitamin A on cytokine secretion by mononuclear cells of preterm newborns and adults.
Neonatology 2007;91:196-202.DOI:
10.1159/000097453. PMID:
17377406.
85. Ehmedah A, Nedeljkovic P, Dacic S, Repac J, Draskovic PB, Vucevic D, et al. Vitamin B complex treatment attenuates local inflammation after peripheral nerve injury.
Molecules 2019;24:4615.DOI:
10.3390/molecules24244615. PMID:
31861069. PMCID:
PMC6943485.
87. Chen J, Tang Z, Slominski AT, Li W, Żmijewski MA, Liu Y, et al. Vitamin D and its analogs as anticancer and anti-inflammatory agents.
Eur J Med Chem 2020;207:112738.DOI:
10.1016/j.ejmech.2020.112738. PMID:
32829183.
89. Shishavan NG, Gargari BP, Kolahi S, Hajialilo M, Jafarabadi MA, Javadzadeh Y. Effects of vitamin K on matrix metalloproteinase-3 and rheumatoid factor in women with rheumatoid arthritis: a randomized, double-blind, placebo-controlled trial.
J Am Coll Nutr 2016;35:392-8.DOI:
10.1080/07315724.2015.1026004. PMID:
26156560.
90. Choi EY, Kim HJ, Han JS. Anti-inflammatory effects of calcium citrate in RAW 264.7 cells via suppression of NF-κB activation.
Environ Toxicol Pharmacol 2015;39:27-34.DOI:
10.1016/j.etap.2014.11.002. PMID:
25434759.
91. Chacko SA, Song Y, Nathan L, Tinker L, de Boer IH, Tylavsky F, et al. Relations of dietary magnesium intake to biomarkers of inflammation and endothelial dysfunction in an ethnically diverse cohort of postmenopausal women.
Diabetes Care 2010;33:304-10.DOI:
10.2337/dc09-1402. PMID:
19903755. PMCID:
PMC2809271.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download