Abstract
Intracranial hemorrhage (ICH) following liver transplantation is a potentially devastating complication. Although hypertension and thrombocytopenia are well-known risk factors for ICH in the general population, their roles in ICH after liver transplantation have not been well established. ICH occurred in two patients with alcoholic cirrhosis after deceased donor liver transplantation. A 38-year-old man presented with acute ICH in the right parietal lobe on day 16 after transplantation, with decreased level of consciousness and seizure. His mental status improved with immediate neurological treatment without surgery. In the second case, a 42-year-old woman had acute ICH in the left frontoparietal lobes on day 9 after transplantation, with generalized tonic-clonic seizures. Urgent cerebral decompression was performed. The patient’s neurological symptoms gradually recovered. In both cases, the blood platelet count was less than 50,000/mm3. Monitoring cerebral pressure for ICH is an invasive and challenging method, especially in patients with cirrhosis who have issues with hemostasis. Surgeons should be critically mindful of the risk of rapid neurological deterioration in patients with cirrhosis. Careful neurologic examination and immediate treatment to lower intracranial pressure for ICH after liver transplantation in patients with alcoholic cirrhosis are very important.
Incidence of neurological complications after liver transplantation (LT) are ranging from 8% to 71%. Seizure is the second most common neurologic complication after LT, with an incidence rate ranging from 3% to 20% [1-3]. Common causes of seizure in liver transplant recipients are metabolic abnormalities, electrolyte imbalance, infection, and immunosuppressant toxicity [4-6]. Recently, a retrospective analysis investigated the incidence of intracranial hemorrhage (ICH) in liver transplant recipients. Among 1,836 adult patients undergoing LT, 36 (2.0%) developed ICH within 30 days. Prolonged intraoperative hypertension and severe thrombocytopenia are associated with postoperative intracerebral hemorrhage [7]. Postoperative blood pressure control and blood platelet levels may be modifiable risk factors for preventing post-transplantation ICH. Monitoring cerebral pressure for ICH is an invasive and challenging method during early LT, especially in patients with cirrhosis who have difficulty with hemostasis. Careful neurologic examinations, which include surveillance of symptomatic changes, such as sudden onset of headache, focal neurologic deficits, and impaired consciousness, are important. This is particularly significant in patients with identified risk factors, high blood sugar level, preoperative abnormal neuroimaging findings, and immediate administration of medication to lower intracranial pressure in patients with alcoholic cirrhosis. Immediate and repeated radiologic examinations are required if decreased level of consciousness, convulsions, or neurological changes occur. We encountered two cases of ICH after LT without any evidence of a structural brain lesion at the beginning of the seizure. The patients were eventually diagnosed with ICH, which was considered to be the starting point for repetitive seizures.
An informed consent form was provided to patients based on the Declaration of Helsinki, and voluntary consent was obtained from all patients. The need for approval from the institutional review board was waived for this study because it is a case report of two patients and does not include protected health information, data analysis, or testing of a hypothesis, and patients were de-identified.
In both cases, preoperative brain computed tomography (CT) was routinely performed to identify abnormal findings such as vascular malformation, hemorrhage, and aneurysm. No abnormal findings were observed. In the first case, a 38-year-old man with alcoholic cirrhosis and hepatic encephalopathy underwent deceased donor LT. His postoperative course was stable, except for the blood platelet count, which was under 50,000/mm3 within 1 week of LT (Table 1). For immunosuppression, FK-506 and steroids were administered. However, there were signs of decreased level of consciousness and weakness of both upper arms on postoperative day 14. Neurological care consultations were also obtained. Neurologic imaging to evaluate brain lesions was performed repeatedly. The patient was diagnosed with acute ICH in the right parietal lobe on day 16 after transplantation (Fig. 1). The patient improved after medication, which lowered intracranial pressure without surgery. He recovered without any neurological sequelae and was discharged from the hospital 1 month postoperatively. The second case was that of a 42-year-old woman with alcoholic cirrhosis, hepatic encephalopathy, and hepatorenal syndrome. Continuous renal replacement therapy (CRRT) was performed before LT owing to the deterioration of renal function. Blood pressure was stable during LT and within 10 days after the operation; thus, CRRT was discontinued. Her blood platelet count was less than 50,000/mm3 within 2 days after LT (Table 2). She had decreased level of consciousness and generalized tonic-clonic seizures on day 9 after transplantation. Neurosurgical and neurological care consultations were performed. Neurological examination included surveillance of symptomatic and mental change and brain CT to assess for positive findings. The patient was diagnosed with acute ICH in the left frontoparietal lobes and underwent emergency craniectomy with drainage (Fig. 2). There were no immediate complications after craniectomy, but neurological sequelae, such as speech impairment and right upper and lower extremity paralysis, remained. After transplantation and craniectomy, serum liver chemistries and tacrolimus trough levels were maintained for 2 months within normal range. After discharge from the hospital, she did not regularly visit outpatient clinics and did not take immunosuppressants regularly. Hyperbilirubinemia developed 4 months post-transplantation, and acute rejection was suspected (Fig. 3). However, the patient refused hospitalization and liver biopsy even though we explained the risks of acute rejection, including graft loss and high morbidity owing to liver failure. We persuaded the patient to take tacrolimus only and attend regular outpatient follow-ups. Finally, graft liver function improved at 9 months postoperatively (Fig. 3). Through continuous rehabilitation treatment, paralysis of the right upper and lower extremities and speech impairment improved.
Owing to various causes, it has been reported that the mortality rate after LT owing to alcoholic liver cirrhosis is high [8]. In a total of 11,590 liver transplant patients, hepatic death occurred in 65%–67% of cases, and cirrhosis or alcoholic liver disease was associated with higher overall mortality. ICH following LT is a potentially devastating complication. The overall incidence is ill-defined, and there are limited reports in the literature regarding etiology and risk factors [9,10]. The cause of ICH may be antiplatelet medication and postoperative coagulopathy owing to post-transplantation liver dysfunction [11]. Thrombocytopenia is a typical feature of chronic liver disease and may worsen intracranial bleeding. While early platelet transfusion should be considered, the threshold for platelet transfusion remains widely debated. A platelet threshold as low as 60×109/L in patients with cirrhosis has been shown to be capable of preserving thrombin generation equivalent to that of healthy participants [12]. Postoperative blood pressure control and pre-transplant fibrinogen levels may be modifiable risk factors for preventing post-transplant ICH. The overall incidence of ICH within 12 months of LT in this cohort was 5.2%. The 30-day and 1-year mortality rates after LT in patients with ICH were 33.3% and 53.3%, respectively. Female sex and higher pre-transplant serum bilirubin levels were associated with intraparenchymal hemorrhage [13]. Transcranial B-mode ultrasound may be useful as a monitoring tool in selected patients, and it also provides early clinical indications of the onset of ICH even before the development of intracranial hypertension or focal neurological deficits [14]. As shown in our case, spontaneous ICH may be followed by alcohol-related cirrhosis with preoperative thrombocytopenia, coagulopathy, and perioperative period. The patient had no history of hypertension or positive brain CT findings. In addition, our case showed that the onset of hemorrhage occurred within 30 days after LT with coagulation disorders. With respect to these findings, we could emphasize that the postoperative routine examination of brain CT or magnetic resonance angiography in alcoholic patients is helpful for the early detection of hemorrhage. Alcoholic cirrhosis has been associated with ICH of the cortex, basal ganglia, pons, and cerebellum but not with medullary hemorrhage. Clinicians should be mindful of the risk of rapid deterioration as intracranial bleeding quickly worsens owing to hemostatic instability in patients with cirrhosis. The lack of sufficient studies has made it difficult to establish clear criteria for ICH evacuation [15,16]. For decades, the role of surgery in ICH and whether hemorrhage evacuation can improve clinical outcomes have been a topic of intense debate. However, in selected patients with hemorrhages and rapid progression of neurological deficits, surgical evacuation can be lifesaving [17]. Urgent surgical management is recommended to reduce mortality and morbidity. Undoubtedly, increased medical comorbidities that are present in the underserved population result in an urgent need for multidisciplinary medical management. Timely consideration of the possibility of rapid deterioration from coagulopathic intracranial bleeding in the initial assessment of alcoholic patients should be maintained. Neurosurgical and neurocritical care consultations were performed for each case of hemorrhage. Repeat neuroimaging was performed to evaluate hemorrhage evolution.
Although ICH is rare, considering that liver transplant recipients have a high risk of bleeding, the evaluation for ICH should be promptly performed when neurological symptoms develop or when mental recovery is slow. Postoperative blood pressure control and blood platelet levels may be modifiable risk factors for preventing post-transplantation ICH. Monitoring cerebral pressure for ICH is an invasive and challenging method, especially in patients with cirrhosis who have difficulty with hemostasis. Thus, surgeons should be critical and scrupulous with their care and be aware of the risk of rapid neurological deterioration in patients with ICH.
ACKNOWLEDGMENTS
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: NL, MY. Data curation: BN, JIL. Formal analysis: MY. Methodology: BN, JIL, MY. Project administration: BN, MY. Visualization: BN, NL. Writing–original draft: BN, MY. Writing–review & editing: MY.
REFERENCES
1. Menegaux F, Keeffe EB, Andrews BT, Egawa H, Monge H, Concepcion W, et al. 1994; Neurological complications of liver transplantation in adult versus pediatric patients. Transplantation. 58:447–50. DOI: 10.1097/00007890-199408270-00010. PMID: 8073514.

2. Bronster DJ, Emre S, Boccagni P, Sheiner PA, Schwartz ME, Miller CM. 2000; Central nervous system complications in liver transplant recipients: incidence, timing, and long-term follow-up. Clin Transplant. 14:1–7. DOI: 10.1034/j.1399-0012.2000.140101.x. PMID: 10693627.
3. Ghaus N, Bohlega S, Rezeig M. 2001; Neurological complications in liver transplantation. J Neurol. 248:1042–8. DOI: 10.1007/s004150170023. PMID: 12013580.

4. Chabolla DR, Wszolek ZK. 2006; Pharmacologic management of seizures in organ transplant. Neurology. 67(12 Suppl 4):S34–8. DOI: 10.1212/WNL.67.12_suppl_4.S34. PMID: 17190920.

5. Mirski MA, Varelas PN. 2008; Seizures and status epilepticus in the critically ill. Crit Care Clin. 24:115–47. ixDOI: 10.1016/j.ccc.2007.11.005. PMID: 18241782.

6. Shepard PW, St Louis EK. 2012; Seizure treatment in transplant patients. Curr Treat Options Neurol. 14:332–47. DOI: 10.1007/s11940-012-0180-y. PMID: 22660960. PMCID: PMC3656593.

7. Gao W, Li J, Nguyen-Buckley C, Nguyen-Lee J, Wray C, Agopian V, et al. 2020; Intraoperative hypertension and thrombocytopenia associated with intracranial hemorrhage after liver transplantation. Transplantation. 104:535–41. DOI: 10.1097/TP.0000000000002899. PMID: 31397798.

8. Yoon J, Jung Y, Kim H, Park B, Choi D. 2020; Cause-specific mortality and associated factors related to death after kidney and liver transplantation: a Korean nationwide study. Korean J Transplant. 34(Suppl 1):S119. DOI: 10.4285/ATW2020.OR-1070.

9. Wang WL, Yang ZF, Lo CM, Liu CL, Fan ST. 2000; Intracerebral hemorrhage after liver transplantation. Liver Transpl. 6:345–8. DOI: 10.1053/lv.2000.6138. PMID: 10827237.

10. Wijdicks EF, de Groen PC, Wiesner RH, Krom RA. 1995; Intracerebral hemorrhage in liver transplant recipients. Mayo Clin Proc. 70:443–6. DOI: 10.4065/70.5.443. PMID: 7731253.

11. Oh SY, Lee H, Park YH, Ryu HG. 2016; Intracranial hemorrhage induced uncontrolled seizure in a deceased donor liver transplant patient: a case report. Korean J Anesthesiol. 69:527–31. DOI: 10.4097/kjae.2016.69.5.527. PMID: 27703637. PMCID: PMC5047992.

12. Takahashi K, Nagai S, Safwan M, Liang C, Ohkohchi N. 2018; Thrombocytopenia after liver transplantation: should we care? World J Gastroenterol. 24:1386–97. DOI: 10.3748/wjg.v24.i13.1386. PMID: 29632420. PMCID: PMC5889819.

13. Gallagher TK, Thomas KA, Ladner DP, Ganger D, Sorond FA, Prabhakaran S, et al. 2018; Incidence and risk factors of intracranial hemorrhage in liver transplant recipients. Transplantation. 102:448–53. DOI: 10.1097/TP.0000000000002005. PMID: 29189631. PMCID: PMC5820203.

14. Bianchini A, DʼAndrea R, Lepic B, Querci L, Laici C, Siniscalchi A. 2019; Intracranial hemorrhage diagnosed with transcranial ultrasound in a comatose, postliver transplant patient. J Stroke Cerebrovasc Dis. 28:104357. DOI: 10.1016/j.jstrokecerebrovasdis.2019.104357. PMID: 31495670.

15. Steiner T, Al-Shahi Salman R, Beer R, Christensen H, Cordonnier C, Csiba L, et al. 2014; European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke. 9:840–55. DOI: 10.1111/ijs.12309. PMID: 25156220.

16. Toyoda K, Steiner T, Epple C, Kern R, Nagayama M, Shinohara Y, et al. 2013; Comparison of the European and Japanese guidelines for the acute management of intracerebral hemorrhage. Cerebrovasc Dis. 35:419–29. DOI: 10.1159/000351754. PMID: 23712243.

17. Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al. 2015; Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 46:2032–60. DOI: 10.1161/STR.0000000000000069. PMID: 26022637.

Fig. 1
Initial magnetic resonance imaging and interval computed tomography images of a 38-year-old man. (A, B) Acute intracranial hemorrhage (ICH) in the right parietal lobe on postoperative day 16. (C, D) Improvement in acute ICH in the right parietal lobe on postoperative month 6.

Fig. 2
Interval computed tomography images of a 42-year-old woman. (A) Acute intracranial hemorrhage (ICH) in the left front-parietal lobes on postoperative day (POD) 9. (B) After craniectomy with drainage for acute ICH in the left front-parietal lobes on POD 15. (C) Improvement in acute ICH after cranioplasty in the left front-parietal lobes on postoperative month 31.
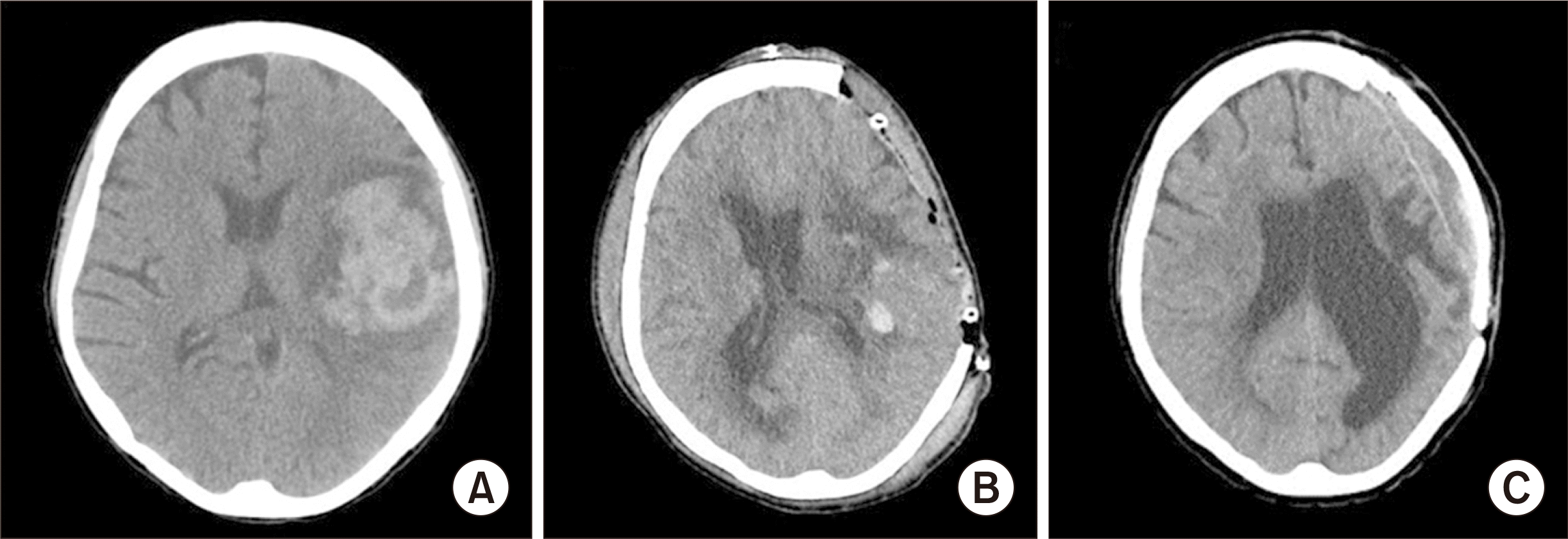
Fig. 3
Serum liver chemistries and tacrolimus level during the transplantation period. The X axis represents various time points after transplantation when the serum liver chemistries and tacrolimus levels were measures (Y axis). Hyper bilirubinemia developed 4 months posttransplantation and improved 9 months postoperatively. AST, aspartate aminotransferase; ALT, alanine transferase.

Table 1
Blood platelet and hemoglobin levels and irradiated platelet pheresis and red cell transfusion in a 38-year-old man
Table 2
Blood platelet and hemoglobin levels and irradiated platelet pheresis and red cell transfusion in a 42-year-old woman




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download