This article has been
cited by other articles in ScienceCentral.
Abstract
The impact of the coronavirus disease 2019 (COVID-19) vaccination on humoral and cellular immunity in transplant recipients remains unknown. We report the case of a 78-year-old kidney transplant recipient who experienced acute T cell-mediated rejection after receiving the second dose of the BNT162b2 mRNA COVID-19 vaccine (Pfizer-BioNTech). She had no history of acute rejection throughout the 13 years after deceased donor kidney transplantation. Fifteen days after receiving the second dose of the BNT162b2 vaccine, the recipient visited our center with a mild headache and fever. Her serum creatinine level had increased from 0.61 to 4.95 mg/dL. Kidney allograft biopsy indicated acute T cell-mediated rejection (grade IB) with no pathologic evidence of antibody-mediated rejection. Anti-severe acute respiratory syndrome coronavirus 2 spike-immunoglobulin G and -immunoglobulin M measurements were weak positive and negative, respectively. Careful monitoring of kidney allograft function is vital for transplant recipients undergoing COVID-19 vaccination.
Go to :

Keywords: Kidney transplant, COVID-19 vaccination, Acute T cell-mediated rejection, Case report
|
HIGHLIGHTS |
There are a few reports about acute rejection after coronavirus disease 2019 (COVID-19) vaccination in kidney transplantation. To our knowledge, this is the first report about acute T cell-mediated rejection after COVID-19 vaccination in a Korean kidney transplant recipient.
|
Go to :

INTRODUCTION
The high infectivity and pathogenicity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to a worldwide pandemic ongoing since January 2020. Symptoms of SARS-CoV-2 infection vary from subclinical course to acute respiratory distress syndrome necessitating mechanical ventilation and extracorporeal membrane oxygenation [
1]. Given the high risk of morbidity and mortality in kidney transplant recipients after SARS-CoV02 infection, earlier administration of coronavirus disease 2019 (COVID-19) vaccines is essential in these individuals [
2-
4]. However, data concerning the humoral and cellular responses after COVID-19 vaccination are limited, and safety outcomes have not been well explored in this population. Here we report the case of a kidney transplant recipient who developed acute T cell-mediated rejection (TCMR) after COVID-19 vaccination.
Go to :

CASE REPORT
The Institutional Review Board of Asan Medical Center approved this study (IRB No. 2021-0101). Written informed consent was achieved from the patient.
The reported clinical activities are consistent with the principles of Declaration of Istanbul on Organ Trafficking and Transplant Tourism. We report the case of a 78-year-old kidney transplant recipient who experienced acute TCMR after receiving the second dose of the BNT162b2 mRNA COVID-19 vaccine (Pfizer-BioNTech; Pfizer, New York, NY, USA). The recipient underwent deceased donor kidney transplantation for hypertension 13 years ago, and had not experienced any adverse event after transplantation except for herpes zoster infection in the early posttransplant period. Her maintenance immunosuppressants were tacrolimus, azathioprine, and low-dose steroids. The patient’s level of serum creatinine 1 month before vaccination was 0.61 mg/dL and the tacrolimus trough level was maintained at 4–5 ng/mL.
Fifteen days after receiving the second dose of the BNT162b2 vaccine, the recipient visited our center with a mild headache and fever. Serum creatine level had increased from 0.61 to 4.95 mg/dL (
Fig. 1), and considerable swelling of the transplanted kidney on non-enhanced computerized tomography was observed. Kidney biopsy revealed acute TCMR (grade IB) with a lack of pathologic evidence of antibody-mediated rejection (
Fig. 2). The allograft biopsy specimen was evaluated for histologic characteristics according to the Banff 2019 criteria as follows: i3, t3, ti3, v0, g1, ci1, ct1, cg0, ptc0, mm1, cv1, ah0, i-IFTA2, t-IFTA3, ptc0, c4d0, aah0, pvl0 [
5]. Luminex single-antigen bead flow assay did not reveal donor-specific anti- human leukocyte antigen antibodies. Anti-SARS-CoV-2 spike immunoglobulin G (IgG) and IgM antibodies (S1-IgG and S1-IgM) were measured using an enzyme-linked immunosorbent assay on the day of the kidney biopsy (18 days after the second vaccination), which revealed S1-IgG and S1-IgM levels of 2.80 (weak positive) and 0.16 (negative), respectively. The patient was administered with steroid pulses (500 mg/day) for 5 days. After 1 month her serum creatinine level had decreased to 2.47 mg/mL.
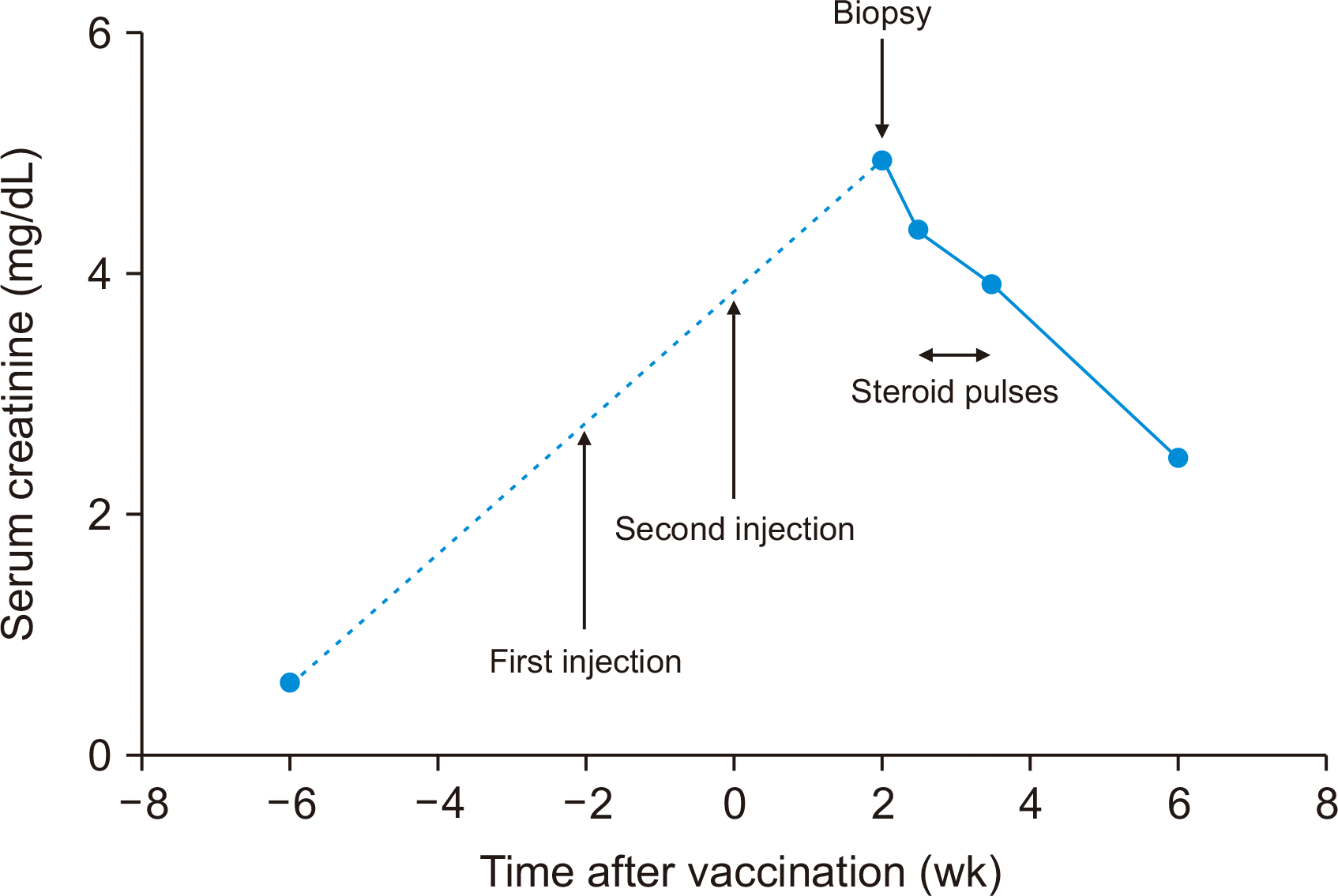 | Fig. 1Changes of serum creatinine according to times after coronavirus disease 2019 (COVID-19) vaccination and steroid pulses. 
|
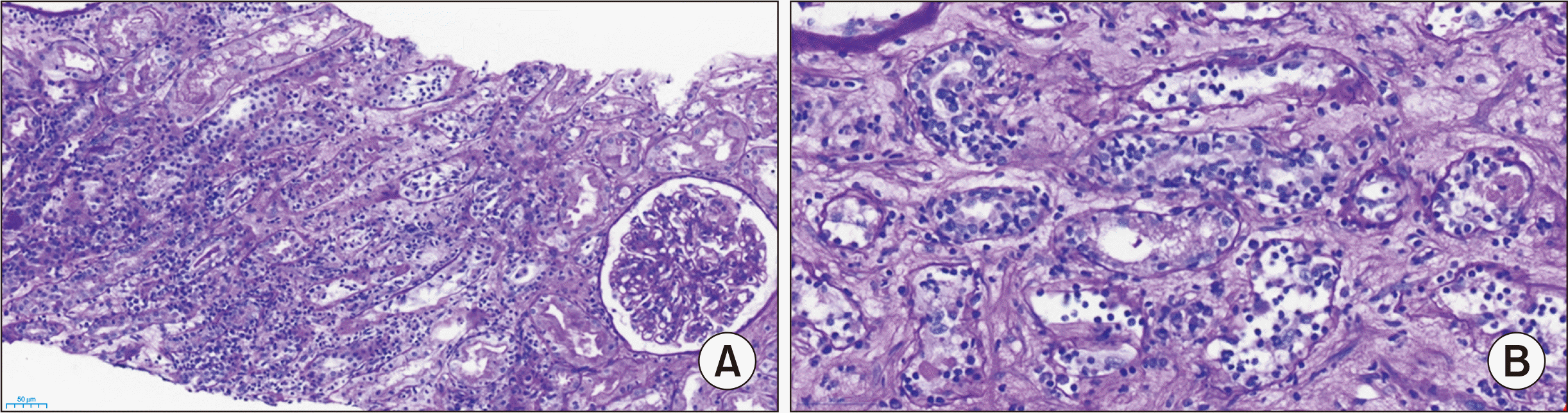 | Fig. 2Hematoxylin and eosin (A; ×200) and periodic acid-Schiff (B; ×400) staining of a kidney allograft with acute T cell-mediated rejection (grade IB). Both stainings showed severe tubulitis and interstitial inflammation. The allograft biopsy specimen was evaluated for histologic characteristics according to the Banff 2019 criteria as follows: i3, t3, ti3, v0, g1, ci1, ct1, cg0, ptc0, mm1, cv1, ah0, i-IFTA2, t-IFTA3, ptc0, c4d0, aah0, pvl0. 
|
Go to :

DISCUSSION
Considering the higher risk of severe pneumonia in SARS-CoV-2-infected patients with comorbidities, guidelines of American Society of Transplantation strongly recommend the SARS-CoV-2 vaccination for transplant recipients [
6,
7]. However, immunosuppressed patients have been excluded from all ongoing SARS-CoV-2 mRNA clinical trials, so data on safety outcomes after vaccination in this population are limited [
8,
9]. Furthermore, data are lacking on the immunologic responses after SARS-CoV-2 vaccination in transplant recipients who remain on immunosuppressants.
Recently, Del Bello et al. [
10] reported on the case of a 23-year-old kidney transplant recipient who had transplantation 18 months prior and presented with acute cellular rejection after the second dose of the BNT162b2 mRNA COVID-19 vaccine (Pfizer-BioNTech). According to the Banff 2019 criteria, interstitial and tubular inflammation were more severe in our case; however, the patient was much older and kidney transplantation had occurred 13 years prior to vaccination. Additionally, Ou et al. [
11] reported that among 741 kidney transplant recipients who underwent BNT162b2 or mRNA-1273 vaccination, one patient was diagnosed with acute rejection after the second dose. However, histologic and humoral characteristics were not described.
Our data demonstrate that a subgroup of kidney transplant recipients may be at risk for acute rejection after COVID-19 vaccination despite low levels of S1-IgG and S1-IgM. Therefore, close monitoring of the kidney allograft is recommended when a transplant recipient plans to undergo COVID-19 vaccination.
Go to :

ACKNOWLEDGMENTS
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: HWJ, HK, YHK, SHK, SS. Formal Analysis: HWJ, SB, YK, SJL, HEK, SS. Methodology: HWJ, JHJ, HYC, HG, SS. Project administration: HWJ, JHJ, HYC, HG, SS. Writing–original draft: HWJ, HK, YHK, SHK, SS. Writing–review & editing: HWJ, HK, YHK, SHK, SS.
Go to :

REFERENCES
1. Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. 2020; Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 55:105924. DOI:
10.1016/j.ijantimicag.2020.105924. PMID:
32081636. PMCID:
PMC7127800.

2. Jager KJ, Kramer A, Chesnaye NC, Couchoud C, Sánchez-Álvarez JE, Garneata L, et al. 2020; Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 98:1540–8. DOI:
10.1016/j.kint.2020.09.006. PMID:
32979369. PMCID:
PMC7560263.

3. Kates OS, Haydel BM, Florman SS, Rana MM, Chaudhry ZS, Ramesh MS, et al. 2021; Coronavirus disease 2019 in solid organ transplant: a multicenter cohort study. Clin Infect Dis. 73:e4090–9. DOI:
10.1093/cid/ciaa1097. PMID:
32766815. PMCID:
PMC7454362.
4. Ravanan R, Callaghan CJ, Mumford L, Ushiro-Lumb I, Thorburn D, Casey J, et al. 2020; SARS-CoV-2 infection and early mortality of waitlisted and solid organ transplant recipients in England: a national cohort study. Am J Transplant. 20:3008–18. DOI:
10.1111/ajt.16247. PMID:
32780493. PMCID:
PMC7436919.

5. Mengel M, Loupy A, Haas M, Roufosse C, Naesens M, Akalin E, et al. 2020; Banff 2019 meeting report: molecular diagnostics in solid organ transplantation-Consensus for the Banff Human Organ Transplant (B-HOT) gene panel and open source multicenter validation. Am J Transplant. 20:2305–17. DOI:
10.1111/ajt.16059. PMID:
32428337. PMCID:
PMC7496585.

7. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. 2020; Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 382:1708–20. DOI:
10.1056/NEJMoa2002032. PMID:
32109013. PMCID:
PMC7092819.

8. BioNTech SE. Study to describe the safety, tolerability, immunogenicity, and efficacy of RNA vaccine candidates against COVID-19 in healthy individuals. Bethesda, MA: ClinicalTrials.gov;2020.
9. ModernaTX. A study to evaluate efficacy, safety, and immunogenicity of mRNA-1273 vaccine in adults aged 18 years and older to prevent COVID-19. Bethesda, MA: ClinicalTrials.gov;2020.
10. Del Bello A, Marion O, Delas A, Congy-Jolivet N, Colombat M, Kamar N. 2021; Acute rejection after anti-SARS-CoV-2 mRNA vaccination in a patient who underwent a kidney transplant. Kidney Int. 100:238–9. DOI:
10.1016/j.kint.2021.04.025. PMID:
33932459. PMCID:
PMC8080493.

11. Ou MT, Boyarsky BJ, Motter JD, Greenberg RS, Teles AT, Ruddy JA, et al. 2021; Safety and reactogenicity of 2 doses of SARS-CoV-2 vaccination in solid organ transplant recipients. Transplantation. 105:2170–4. DOI:
10.1097/TP.0000000000003780. PMID:
33859151. PMCID:
PMC8487696.

Go to :







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download