This article has been
cited by other articles in ScienceCentral.
Abstract
Background
As a solution to organ shortages, studies on kidney transplantation (KT) from older donors are being conducted. However, many controversies remain about its safety and efficacy.
Methods
In Samsung Medical Center, from January 2000 to May 2015, 1,141 patients underwent living KT. Cases of retransplantation, recipient and donor aged younger than 18 years, and multiorgan transplantation were excluded, and a total of 859 cases were selected. Analysis was performed by dividing the patents into two groups a younger donor group (donors <60 years old; n=826) and an older donor group (donors ≥60 years old; n=33).
Results
There were no significant differences between the two groups in patient death (log-rank P=0.173) or in postoperative complications. The older donor group had a higher acute rejection (P=0.034; hazard ratio [HR], 1.704) and graft failure rate (P=0.029, HR=2.352). There was no significant difference in the trend of estimated glomerular filtration rate over time (P=0.189).
Conclusions
KT using kidneys from old-aged donors is safe, but there is room for improvement due to problems with higher acute rejection and graft failure rate.
Keywords: Kidney transplantation, Living donors, Aged
|
HIGHLIGHTS |
Organ shortage has been a big issue in kidney transplantation. Postop complication and estimated glomerular filtration rate over time had no significant difference between the two groups. Older donor kidney transplantation could be a solution to organ shortage.
|
INTRODUCTION
Kidney transplantation (KT) is the best treatment for patients with end-stage kidney disease [
1]. As the importance of KT is increasingly emphasized, the demand for KT and the number of kidney transplants are also increasing. According to Korean Network for Organ Sharing (KONOS) data, the number of kidney transplant cases in 2018 increased by 1.7 times from the number in 2009 (from 1,238 to 2,108 cases).
Supply is also increasing as demand increases but at much slower pace, so the number of people waiting for transplantation and the average waiting period are increasing every year. The number of people waiting increased by about 2.7 times from 8,488 in 2009 to 22,620 in 2018. The average waiting time also increased by about 1.3 times to 5.6 years in 2018, compared to 4.4 years in 2009. This suggests that organ shortage is progressing due to an imbalance between supply and demand.
As a solution to this organ shortage, KT using older kidney donors has been proposed over a decade. In fact, as the average age of donors in living KT is increasing, various studies on these solutions are being conducted. Most of the previous studies showed that there is no significant difference in kidney function and safety between the two groups (recipient of older donor and recipient of younger donor) and drew conclusions that would recommend KT using a kidney from an old living donor, but each study has limitations. They either had too small of a study population enrolled, or the follow-up period was not long enough, ranging from 1 to 3 years [
1,
2]. Some included hypertension (HTN), diabetes mellitus (DM), body mass index (BMI), albuminuria, etc., as well as the age variable through the definition of marginal donor, so the relationship with age was unclear [
1]. Some studies had a large enough population and a long-term follow-up period, and the result showed that the outcome from older living donor KT (LDKT) was superior to that of deceased donor KT (DDKT), but compared to younger donor living KT, the conclusion was not satisfactory for older donor transplantation, and the solution for improvement of the unsatisfactory was not considered [
3]. There was another study in which only donor safety was analyzed and the efficacy aspect was not mentioned [
4]. In this paper, we attempted to investigate the effectiveness and safety of older kidney donors, especially in living KT only, with a follow-up period of 5 years, as a solution to organ shortage problems in KT, and tried to come up with the improvements for the problems that older donor KT might have.
METHODS
Study Population
We retrospectively reviewed 1,684 patients who underwent KT from January 1, 2000 to May 29, 2015 from Samsung Medical Center (SMC). The study protocol was reviewed and approved by the Institutional Review Board at SMC (IRB No. 2020-04-037-003; as a retrospective study using already collected data, it was a study that did not require the subject’s consent). Chart review was conducted through electronic medical record for the patients undergoing their first KT, who were 18 years of age or older and who followed up for 5 years or more. Of these, 1,141 cases of LDKT were included, and 226 cases of donor and recipient being under 18 years of age, multiorgan transplantation, retransplantation, or the follow-up period being less than 5 years were excluded from the review. Two cases with insufficient data and seven cases of daclizumab, horse antithymocyte globulin (ATG), rituximab+simulect induction and 47 cases of ABO incompatible transplantation were also excluded. According to the above inclusion and exclusion criteria, a total of 859 people were included in the study population, which was divided into two groups: younger donor group with donors aged 18 to 59 (n=826) and an older donor group with donors aged 60 and over (n=33) (
Fig. 1). Of these patients, overall patient characteristics and recipient outcome was analyzed in terms of safety and efficacy. For safety, death rate and post op complications during the hospitalized period and for efficacy, death censored graft failure rate, acute rejection rate (biopsy proven) and estimated glomerular filtration rate (eGFR) over time were analyzed as primary outcome. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.
Immunosuppression and Posttransplantation Management
For induction therapy, rabbit ATG (rATG, 1.5 mg/kg, three doses on days 0, 1, and 2) or interleukin-2 receptor antagonist (basiliximab, 20 mg/kg, two doses on days 0 and 4) was used. In addition, a single dose of anti-CD20 monoclonal antibody on day 0 (rituximab, 375 mg/m2) was used for recipients with donor-specific anti-human leukocyte antigen (HLA) antibodies (DSAs). Induction therapy was mainly decided according to the immunologic risk and physician’s preference. Calcineurin inhibitor (tacrolimus or cyclosporine), mycophenolate mofetil, and prednisone were used for maintenance therapy. Tacrolimus was replaced with sirolimus when the BK virus DNA load was >4 log copies/mL.
Renal artery stenosis, ureter leakage, ureter stenosis, postoperative bleeding, wound complications, renal vein thrombosis, and lymphocele were analyzed as postoperative complications. Cases of transfusion after surgery were defined as having post op bleeding and lymphocele was analyzed by defining it as a case where percutaneous catheter drainage was required or discharged without drain removal. To check the complications of the ureter and blood vessels, renal scan with 99mTc-DTPA (Diethylene triamine pentaacetic acid) on day 5 was checked and renal Doppler ultrasound was also routinely checked on days 1 and 5 after transplantation.
Statistical Analysis
Statistical analysis was executed using SAS ver. 9.4 (SAS Institute Inc., Cary, NC, USA) and R 3.6.1 (R Foundation, Vienna, Austria). Differences between the two groups were analyzed using the chi-square test or Fisherʼs exact test for categorical variables, and the t-test or Wilcoxon rank sum test for continuous variables. The association of potential risk factors with outcomes was tested using Cox proportional-hazards regression analysis. Variables with a P-value of less than 0.1 in the univariable analysis were included in the multivariable analysis. Multicollinearity was checked using the variance inflation factor (VIF). There are no variables with VIF >4. Logarithmic transformation was used for the dialysis duration, which had a skewed distribution. The association of potential risk factors with outcomes was tested using logistic regression analysis. The association of potential risk factors with outcomes was tested using linear regression analysis. Analysis using the Generalized Estimating Equation was applied to repeated measurements of parameters.
RESULTS
When the characteristics of the younger donor group (donors <60 years) and the older donor group (donors ≥60 years old) were analyzed, there were no significant differences between the two groups in donor sex, BMI, underlying disease, or creatinine level. There was also no significant difference in recipients between the two groups, except that the follow-up period of the older donor group was shorter than that of the younger donor group (
Table 1). This means that there was no significant difference in characteristics between the two groups except for donor age.
Safety of Old Aged Donor Kidney Transplantation
Patient death of recipients after KT was analyzed, and the younger and older donor groups had 31 (3.8%) and two (6.1%) deaths, respectively, during the follow-up period (P=0.279). In the Kaplan-Meier plot, there was no difference in the overall survival graph between the two groups (log-rank P=0.173) (
Supplementary Fig. 1). When risk factor analysis of patient death was performed, using a kidney from and old-aged donor did not act as a significant risk factor for patient death. Multivariable analysis showed that recipient age was the only significant risk factor for patient death (P<0.001; hazard ratio [HR], 1.083) (
Supplementary Table 1).
The incidences of immediate postoperative complications such as renal artery stenosis, ureter leakage, ureter stenosis, postoperative bleeding, wound complications, and lymphocele were not significantly different between the two groups (
Table 2). The incidence of renal vein thrombosis was found to be significantly greater in the older donor group, but there was only one case in each group, so it is necessary to interpret that result cautiously.
Efficacy of Old Aged Donor Kidney Transplantation
When analyzed in the younger donor and older donor groups, the numbers of acute rejections were 323 (39.1%) and 21 (63.6%), respectively, with a higher rate in the older donor group (P=0.002). Multivariable analysis was performed (recipient age, recipient sex, HLA mismatch, DSA positive, underlying kidney disease, donor HTN were treated as a fixed variables), and it was also shown that belonging to the older donor group acts as a risk factor for acute rejection (P=0.034; HR, 1.704) (
Table 3). In the Kaplan-Meier curve representing the rejection-free survival rate for 10 years, significantly poor results were also found in the older donor group (log-rank P=0.002) (
Fig. 2A).
The age of the donor was also a risk factor for graft failure when multivariable analysis (recipient age, recipient BMI, recipient DM were treated as a fixed variables) was performed (P=0.029; HR, 2.352) (
Table 4). The Kaplan-Meier curve in
Fig. 2B also confirms that the graft survival rate was significantly worse in the older group (log-rank P=0.026). The mean value of 10-year eGFR was significantly lower in the older group (77.75 vs. 60.30). Nevertheless, eGFR interaction over time was not significantly different between the two groups (P=0.189); the trend of change in eGFR over time is also shown as mean plot (
Fig. 3).
DISCUSSION
According to the analyzed results so far, there were no significant difference in postoperative complications or patient death between the two groups, so it was confirmed that there was no significant problem in terms of safety. KT from an older kidney donor acted as a significant risk factor for graft failure and acute rejection, but it was confirmed that there was no significant difference between the two groups in the trend of eGFR over time. It can be analyzed as a result showing that some supplementation is needed in terms of efficacy.
In older donor KT, it would also be important to perform optimal immunosuppression. Since older donor kidneys are generally immunogenic, KT with an older donor kidney tends to have a higher rejection rate [
5-
7]. Thus, immunosuppression agents that have better anti-rejection effects should be selected at the appropriate dose for older donor kidneys. For example, there are studies that show better outcomes of rATG induction compared to basiliximab induction, in terms of the anti-rejection effect [
8-
10]. To reduce the rejection rate induced by the immunogenicity of older donor kidneys, rATG induction may be a better choice.
Older donor kidneys are known to be more vulnerable to histologic damage [
11] and have an impaired ability to restore tissue from damage [
6]. Therefore, it is important to avoid nephrotoxic agents, especially in KT with older kidney donors. In fact, several studies confirmed that nephrotoxicity was worse in the aged group in DDKT [
12,
13]. In particular, calcineurin inhibitor (CNI) is a good immunosuppressive agent and is widely used, but it has nephrotoxicity [
14], so applying a strategy for withdrawal, minimization or avoidance may be helpful [
15]. In addition, Xia et al. [
16] concluded that older donor kidney is a risk factor for CNI nephrotoxicity. In expanded criteria donor (ECD), delayed CNI introduction through rATG induction or use of CNI-free regimens such as mammalian target of rapamycin inhibitor-based regimens may be considered [
17]. In this way, additional studies to improve the efficacy of old donor LDKT should be conducted by borrowing the concepts of studies conducted in ECD KT.
As a solution to the aforementioned problems in terms of efficacy, the concept of old-for-old kidney allocation could be considered. Older kidney grafts tend to have shorter graft survivals and lower eGFR, and are more susceptible to ischemic damage, making them more prone to graft loss. This method is used because there is a higher probability of an older recipient dying before the end of the relatively short lifespan of these grafts, thus minimizing graft loss [
18]. Old-for-old kidney allocation in DDKT has been used as a solution to organ shortage since 1999 [
19]. By introducing this concept to LDKT, it could be used to improve graft failure through optimal matching between donors and recipients.
There are several limitations. First, there may be missing information due to data collection occurring through chart review. Second, the data was collected retrospectively by a single institution. Another complementary point is that information on donor safety, such as the donorʼs post-op complications, must be added to evaluate risk benefits. In addition, the fact that the number of patients enrolled in the older donor group is relatively small, suggest the need for a study on a larger population.
According to the analyzed results, there were no significant differences in patient death or post-op complications between the two groups. However, in terms of efficacy, KT using kidneys from aged donors increases the risk of acute rejection (P=0.034; HR, 1.704) and graft failure (P=0.029; HR, 2.352). It seems that a strategy to increase efficacy is needed and selecting optimal immunosuppression and applying old-to-old strategies could serve as effective strategies. Further study is needed to verify this.
ACKNOWLEDGMENTS
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Funding/Support
This study was supported by research grant from the Korean Society for Transplantation (2021-00-01010-021).
Author Contributions
Conceptualization: KWL, JBP. Data curation: JYS, KWL, KDK, JY, JEK, JBP. Formal analysis: JYS, KAK. Methodology: JYS, KWL, KAK. Project administration: JBP. Visualization: KAK. Writing–original draft: JYS, KWL. Writing–review & editing: JYS, KWL, OL, JBP.
REFERENCES
1. Oikawa M, Hatakeyama S, Narita T, Yamamoto H, Hosogoe S, Imai A, et al. 2016; Safety and effectiveness of marginal donor in living kidney transplantation. Transplant Proc. 48:701–5. DOI:
10.1016/j.transproceed.2015.09.067. PMID:
27234717.

3. Gill JS, Gill J, Rose C, Zalunardo N, Landsberg D. 2006; The older living kidney donor: part of the solution to the organ shortage. Transplantation. 82:1662–6. DOI:
10.1097/01.tp.0000250715.32241.8a. PMID:
17198256.

4. Toyoda M, Yamanaga S, Kawabata C, Hidaka Y, Inadome A, Arakane F, et al. 2014; Long-term safety of living kidney donors aged 60 and older. Transplant Proc. 46:318–20. DOI:
10.1016/j.transproceed.2013.11.019. PMID:
24655952.

6. Fijter JW, Mallat MJ, Doxiadis II, Ringers J, Rosendaal FR, Claas FH, et al. 2001; Increased immunogenicity and cause of graft loss of old donor kidneys. J Am Soc Nephrol. 12:1538–46. DOI:
10.1681/ASN.V1271538. PMID:
11423584.

7. Colvin MM, Smith CA, Tullius SG, Goldstein DR. 2017; Aging and the immune response to organ transplantation. J Clin Invest. 127:2523–9. DOI:
10.1172/JCI90601. PMID:
28504651. PMCID:
PMC5490761.

8. Ulrich F, Niedzwiecki S, Pascher A, Kohler S, Weiss S, Fikatas P, et al. 2011; Long-term outcome of ATG vs. Basiliximab induction. Eur J Clin Invest. 41:971–8. DOI:
10.1111/j.1365-2362.2011.02490.x. PMID:
21382021.

9. Hardinger KL, Brennan DC, Klein CL. 2013; Selection of induction therapy in kidney transplantation. Transpl Int. 26:662–72. DOI:
10.1111/tri.12043. PMID:
23279211.

10. Bächler K, Amico P, Hönger G, Bielmann D, Hopfer H, Mihatsch MJ, et al. 2010; Efficacy of induction therapy with ATG and intravenous immunoglobulins in patients with low-level donor-specific HLA-antibodies. Am J Transplant. 10:1254–62. DOI:
10.1111/j.1600-6143.2010.03093.x. PMID:
20353473.

11. Naesens M, Lerut E, de Jonge H, Van Damme B, Vanrenterghem Y, Kuypers DR. 2009; Donor age and renal P-glycoprotein expression associate with chronic histological damage in renal allografts. J Am Soc Nephrol. 20:2468–80. DOI:
10.1681/ASN.2009020192. PMID:
19762492. PMCID:
PMC2799173.

12. Greenfeld Z, Peleg I, Brezis M, Rosen S, Pisanty S. 1994; Potential interaction between prolonged cyclosporin administration and aging in the rat kidney. Ann N Y Acad Sci. 717:209–12. DOI:
10.1111/j.1749-6632.1994.tb12089.x. PMID:
8030836.

13. Legendre C, Brault Y, Morales JM, Oberbauer R, Altieri P, Riad H, et al. 2007; Factors influencing glomerular filtration rate in renal transplantation after cyclosporine withdrawal using sirolimus-based therapy: a multivariate analysis of results at five years. Clin Transplant. 21:330–6. DOI:
10.1111/j.1399-0012.2007.00645.x. PMID:
17488381.

14. Naesens M, Kuypers DR, Sarwal M. 2009; Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 4:481–508. DOI:
10.2215/CJN.04800908. PMID:
19218475.

15. Bhat M, Mara K, Dierkhising R, Watt KD. 2018; Immunosuppression, race, and donor-related risk factors affect de novo cancer incidence across solid organ transplant recipients. Mayo Clin Proc. 93:1236–46. DOI:
10.1016/j.mayocp.2018.04.025. PMID:
30064826.
16. Xia T, Zhu S, Wen Y, Gao S, Li M, Tao X, et al. 2018; Risk factors for calcineurin inhibitor nephrotoxicity after renal transplantation: a systematic review and meta-analysis. Drug Des Devel Ther. 12:417–28. DOI:
10.2147/DDDT.S149340. PMID:
29535503. PMCID:
PMC5836651.

17. Filiopoulos V, Boletis JN. 2016; Renal transplantation with expanded criteria donors: which is the optimal immunosuppression? World J Transplant. 6:103–14. DOI:
10.5500/wjt.v6.i1.103. PMID:
27011908. PMCID:
PMC4801786.

18. Moers C, Kornmann NS, Leuvenink HG, Ploeg RJ. 2009; The influence of deceased donor age and old-for-old allocation on kidney transplant outcome. Transplantation. 88:542–52. DOI:
10.1097/TP.0b013e3181b0fa8b. PMID:
19696638.

19. Fritsche L, Hörstrup J, Budde K, Reinke P, Giessing M, Tullius S, et al. 2003; Old-for-old kidney allocation allows successful expansion of the donor and recipient pool. Am J Transplant. 3:1434–9. DOI:
10.1046/j.1600-6135.2003.00251.x. PMID:
14525606.

Fig. 1
Study population. DDKT, deceased donor kidney transplantation; KT, kidney transplantation; FU, follow-up; hATG, horse antithymocyte globulin.
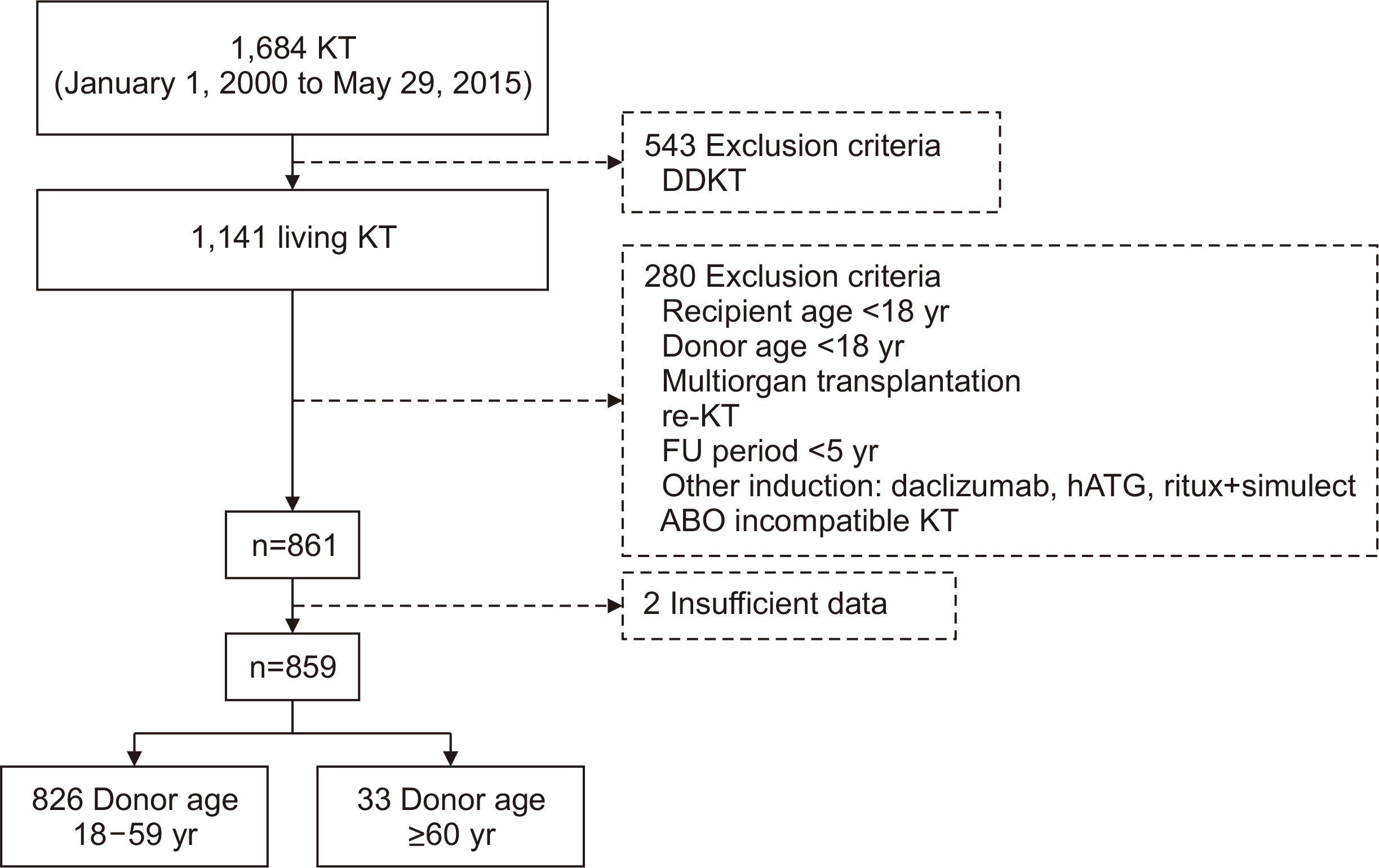
Fig. 2
Rejection and graft loss. (A) Kaplan-Meier curve of acute rejection rate in patients after living kidney transplantation. (B) Kaplan-Meier curve of graft survival rate in patients after living kidney transplantation. Number at risk is also shown below the graph.
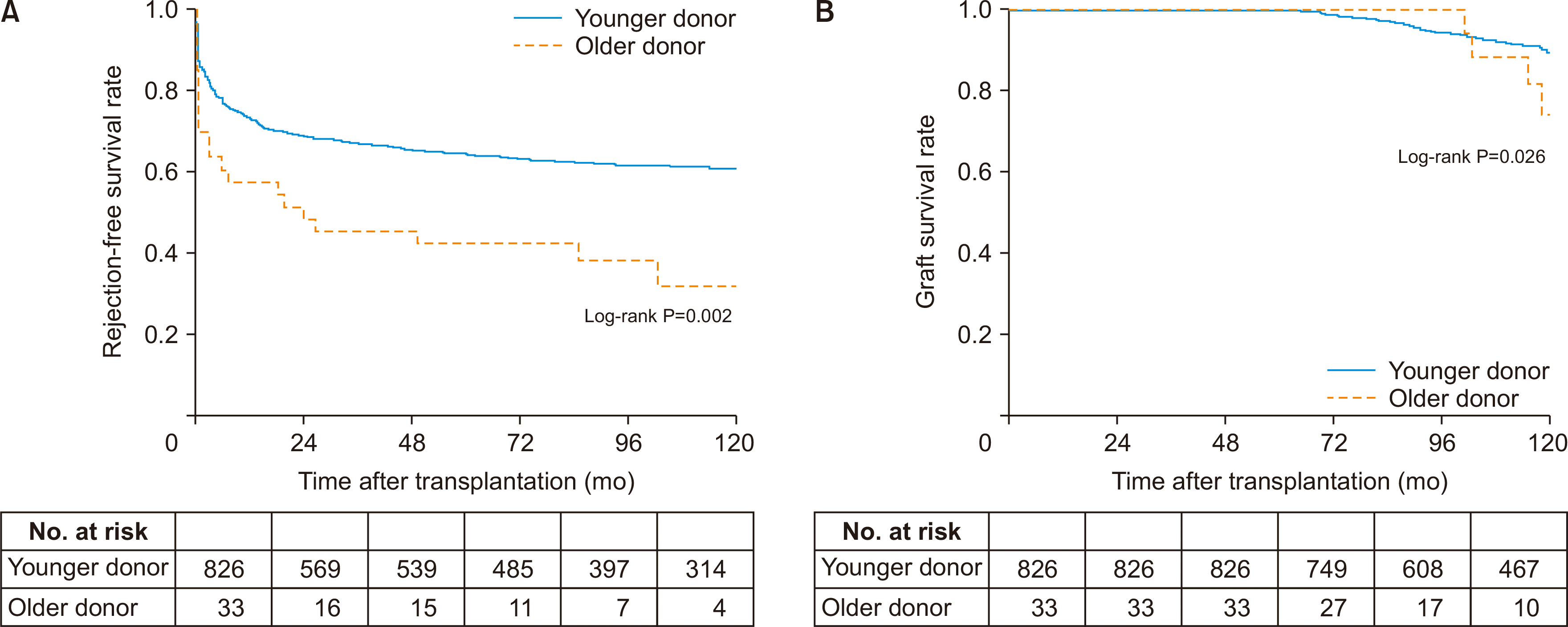
Fig. 3
Estimated glomerular filtration rate (eGFR) over time. Renal function shown as eGFR is lower in the older donor group but the trend over time is not significantly different between the two groups. Number at risk is also shown below the graph.
Table 1
Characteristics of older and younger living donors and recipient
|
Variable |
Younger donora) (n=826) |
Older donorb) (n=33) |
P-value |
|
Donor |
|
|
|
|
Age (yr) |
39.4 |
62.6 |
<0.001 |
|
Female |
398 (48.2) |
15 (45.5) |
0.753 |
|
BMI (kg/m2) |
24.10 |
24.26 |
0.620 |
|
Underlying disease |
|
|
|
|
DM |
5 (0.61) |
0 |
>0.999 |
|
HTN |
24 (2.75) |
2 (6.06) |
0.244 |
|
Creatinine level (mg/dL) |
0.85 |
0.85 |
0.835 |
|
Recipient |
|
|
|
|
Age (yr) |
42.4 |
46.2 |
0.138 |
|
Female |
356 (43.1) |
16 (48.5) |
0.540 |
|
BMI (kg/m2) |
22.53 |
22.97 |
0.411 |
|
Dialysis duration (day) |
697.58 |
579.73 |
0.440 |
|
Number of HLA mismatches |
2.73 |
3.00 |
0.381 |
|
Risk factor |
|
|
|
|
DM |
134 (16.22) |
5 (15.15) |
0.870 |
|
HTN |
679 (82.20) |
26 (78.79) |
0.616 |
|
Underlying kidney disease |
|
|
0.610 |
|
DM nephropathy |
116 (14.04) |
5 (15.15) |
- |
|
GN (IgA, FSGS, Other GN) |
239 (28.93) |
9 (27.27) |
- |
|
ADPCK |
33 (4.00) |
3 (9.09) |
- |
|
Hypertensive |
118 (14.29) |
5 (15.15) |
- |
|
Other and unknown |
320 (38.74) |
11 (33.33) |
- |
|
DSA-positivec)
|
27 (3.43) |
1 (3.45) |
>0.999 |
|
Induction method |
|
|
0.148 |
|
No agent |
380 (46.0) |
10 (30.3) |
- |
|
Basiliximab |
368 (44.55) |
18 (54.55) |
- |
|
r-ATG |
43 (5.21) |
2 (6.06) |
- |
|
r-ATG+rituximab |
35 (4.2) |
3 (9.09) |
- |
|
Maintenance regimen |
|
|
0.441 |
|
CsA+MMF+PD |
340 (41.2) |
10 (30.3) |
- |
|
FK+MMF+PD |
469 (56.8) |
23 (69.7) |
- |
|
Sirolimus or everolimus Combination |
14 (1.69) |
0 |
- |
|
Otherd)
|
3 (0.36) |
0 |
- |
|
Follow-up duration (yr) |
11.19 |
8.99 |
0.002 |
Table 2
Postoperative complications
|
Variable |
Younger donor
(n=826) |
Older donor
(n=33) |
P-value |
|
Renal artery stenosis |
3 (0.4) |
0 |
0.415 |
|
Ureter leakage |
11 (1.3) |
0 |
0.969 |
|
Ureter stenosis |
3 (0.4) |
0 |
0.415 |
|
Renal vein thrombosis |
1 (0.1) |
1 (3) |
0.023 |
|
Others |
2 (0.2) |
0 |
0.313 |
|
Postoperative bleeding |
54 (6.5) |
0 |
0.285 |
|
Wound complicationa)
|
18 (2.2) |
0 |
0.771 |
|
Lymphocele |
34 (4.1) |
1 (3) |
0.758 |
Table 3
Risk factor analysis of acute rejection
|
Risk factor |
Univariable |
|
Multivariablea)
|
|
|
|
HR (95% CI) |
P-value |
HR (95% CI) |
P-value |
|
Recipient |
|
|
|
|
|
|
Age |
0.988 (0.978–0.997) |
0.012 |
|
0.980 (0.969–0.990) |
<0.001 |
|
Male |
1.468 (1.179–1.829) |
0.001 |
|
1.486 (1.176–1.878) |
0.001 |
|
BMI |
1.026 (0.995–1.058) |
0.103 |
|
- |
- |
|
DM |
0.099 (0.740–1.323) |
0.941 |
|
- |
|
|
HTN |
0.903 (0.692–1.177) |
0.451 |
|
|
|
|
Underlying kidney disease 2b)
|
|
0.071 |
|
- |
0.412 |
|
DM nephropathy |
|
ref |
|
- |
|
|
GN (IgA, FSGS, others) |
0.980 (0.702–1.370) |
0.908 |
|
0.924 (0.645–1.325) |
0.669 |
|
ADPCK |
1.461 (0.878–2.429) |
0.144 |
|
1.275 (0.755–2.153) |
0.364 |
|
Hypertensive |
0.883 (0.596–1.307) |
0.534 |
|
0.945 (0.627–1.423) |
0.786 |
|
Other and UK |
0.778 (0.560–1.081) |
0.135 |
|
0.811 (0.571–1.151) |
0.241 |
|
Dialysis duration |
0.976 (0.924–1.031) |
0.382 |
|
- |
- |
|
Pre-emptive KTc)
|
1.015 (0.798–1.29) |
0.905 |
|
- |
- |
|
HLA mismatch |
1.180 (1.103–1.263) |
<0.001 |
|
1.145 (1.065–1.231) |
<0.001 |
|
DSA-positive |
1.701 (1.013–2.856) |
0.045 |
|
2.013 (1.012–4.004) |
0.046 |
|
Induction agent |
|
0.009 |
|
|
0.015 |
|
No agent |
- |
ref |
|
- |
- |
|
Basiliximab |
1.332 (1.061–1.673) |
0.014 |
|
1.257 (0.990–1.597) |
0.061 |
|
r-ATG |
1.958 (1.277–3.004) |
0.002 |
|
1.965 (1.269–3.043) |
0.003 |
|
r-ATG+rituximab |
1.153 (0.676–1.966) |
0.602 |
|
0.984 (0.489–1.982) |
0.964 |
|
Donor |
|
|
|
|
|
|
Male |
0.953 (0.770–1.177) |
0.653 |
|
|
|
|
BMI |
1.018 (0.986–1.052) |
0.270 |
|
|
|
|
DM |
0.925 (0.231–3.704) |
0.912 |
|
|
|
|
HTN |
1.624 (0.913–2.889) |
0.099 |
|
1.326 (0.739–2.379) |
0.344 |
|
Cr |
0.780 (0.406–1.498) |
0.455 |
|
|
|
Older donor groupd)
|
1.999 (1.285–3.109) |
0.002 |
|
1.704 (1.042–2.784) |
0.034 |
Table 4
Risk factor analysis of graft failure
|
Risk factor |
Univariable |
|
Multivariablea)
|
|
|
|
HR (95% CI) |
P-value |
HR (95% CI) |
P-value |
|
Recipient |
|
|
|
|
|
|
Age |
0.980 (0.964–0.996) |
0.015 |
|
0.971 (0.954–0.987) |
0.001 |
|
Male |
1.225 (0.871–1.722) |
0.243 |
|
|
|
|
BMI |
1.061 (1.012–1.112) |
0.015 |
|
1.055 (1.007–1.107) |
0.025 |
|
DM |
1.692 (1.094–2.616) |
0.018 |
|
1.962 (1.225–3.141) |
0.005 |
|
HTN |
0.729 (0.048–1.118) |
0.148 |
|
|
|
|
Underlying kidney disease 2b)
|
|
0.024 |
|
|
|
|
DM nephropathy |
|
ref |
|
|
|
|
GN (IgA, FSGS, others) |
0.481 (0.285–0.810) |
0.006 |
|
|
|
|
ADPCK |
0.701 (0.288–1.709) |
0.435 |
|
|
|
|
Hypertensive |
0.632 (0.362–1.104) |
0.107 |
|
|
|
|
Other and UK |
0.465 (0.287–0.755) |
0.002 |
|
|
|
|
Dialysis duration |
0.962 (0.879–1.054) |
0.408 |
|
|
|
|
Pre-emptive KTc)
|
0.942 (0.621–1.429) |
0.778 |
|
|
|
|
HLA mismatch |
1.027 (0.920–1.146) |
0.641 |
|
|
|
|
DSA positive |
1.071 (0.263–4.352) |
0.924 |
|
|
|
|
Induction agent |
|
0.575 |
|
|
|
|
No agent |
|
ref |
|
|
|
|
Basiliximab |
1.247 (0.805–1.932) |
0.323 |
|
|
|
|
r-ATG |
1.545 (0.618–3.864) |
0.352 |
|
|
|
|
r-ATG+rituximab |
1.656 (0.512–5.361) |
0.400 |
|
|
|
|
Maintenance regimen |
|
0.871 |
|
|
|
|
CsA+MMF+PD |
|
ref |
|
|
|
|
FK+MMF+PD |
0.914 (0.643–1.293) |
0.604 |
|
|
|
|
Sirolimus or everolimus combination |
1.321 (0.369–4.725) |
0.669 |
|
|
|
|
Otherd)
|
1.821 (0.108–30.69) |
0.677 |
|
|
|
|
Donor |
|
|
|
|
|
|
Male |
0.880 (0.631–1.226) |
0.449 |
|
|
|
|
BMI |
1.036 (0.986–1.088) |
0.164 |
|
|
|
|
DM |
1.725 (0.241–12.365) |
0.588 |
|
|
|
|
HTN |
0.569 (0.079–4.078) |
0.575 |
|
|
|
|
Cr |
0.739 (0.254–2.152) |
0.579 |
|
|
|
|
Older donor groupe)
|
2.320 (1.081–4.978) |
0.031 |
|
2.352 (1.093–5.061) |
0.029 |







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download