Abstract
Background
The presence of preformed donor-specific antibodies in recipient serum against anti-human leukocyte antigen is a significant risk factor that negatively affects the outcomes of intestinal transplantation. Avoiding high-risk intestinal transplantation by physical and virtual cross matches has had limited success due to time constraints and ineffective correlation, respectively.
Methods
We developed a guideline to improve the association between physical and virtual cross matches using the retrospective data of 56 consecutive primary adult isolated intestinal transplantations from a single center.
Results
The mean fluorescence intensity of 2,000 for positive donor-specific antibodies revealed the best association between physical and virtual cross matches among different cut-off values, but with an unacceptable false positive rate of 54%. An enhanced virtual cross match with the summation of the mean fluorescence intensity of each anti-human leukocyte antigen improved the association between physical and virtual cross matches, with a sensitivity of 83% and specificity of 98%.
Conclusions
This enhanced virtual cross match more effectively predicts high-risk intestinal transplantation and is a better substitute for physical cross-match than the current virtual cross match. It also helps to avoid ill-considered abandonment of intestinal transplantation that is unnecessarily deemed high risk based on a simple virtual cross match.
The presence of antibodies (Abs) to donor human leukocyte antigen (HLA) is a well-recognized risk factor for the development of acute rejection (AR) after intestinal transplantation (ITX). Physical cross-match (pXM) between recipient serum and donor lymphocytes can be used to identify such Abs and is strongly associated with outcome after ITX [1,2]. Patients with positive pXM, implying the presence of Ab to donor HLA at the time of ITX, are at higher risk of AR and poor graft survival. Despite this strong correlation, pXM is not a practical tool to avoid high-risk ITX because of time constraints, and cold ischemia time usually becomes unacceptably long while waiting for the result of pXM. However, virtual cross match (vXM) only requires serum from the recipient at any time before ITX, and no specimen is necessary from the donor other than for HLA typing, which eliminates the time constraints of pXM. Hence, there is interest in the use of vXM as a potential substitute for pXM. The anti-HLA Ab profile of the recipient can be determined from the recipient’s serum using a single antigen bead assay (SBA). When donor HLA typing is available, usually at the time of organ offer, identification of donor-specific anti-HLA Ab (DSA) can be performed, that is vXM, using the anti-HLA Ab profile of the recipient and HLA typing of the donor. Unlike pXM, this characteristic of vXM allows for the avoidance of a high-risk ITX at the stage of organ offer. However, vXM has drawbacks. Ab activity in SBA is displayed as the mean fluorescence intensity (MFI), and the presence of anti-HLA Ab is confirmed when the MFI is above a certain value. Therefore, the results of vXM will be different in the same sample if a different MFI cutoff value is used. As a corollary, it is possible that the difference will swing between positive and negative. Furthermore, the results of vXM and pXM can differ. Therefore, the clinical relevance of vXM remains limited as a tool to avoid high-risk ITX. We analyzed our clinical experience under a strict and consistent protocol in a relatively homogeneous patient group with prospectively maintained data in an attempt to develop guidelines that provide better clinical association between pXM and vXM.
We performed a retrospective study using a prospectively maintained database to identify effective MFI cutoff values to define DSA and develop relevant clinical guidelines to identify high-risk ITX, using a more effective substitute for vXM than has been previously reported. The study was approved by the Institutional Review Board of Mount Sinai Medical Center (IRB No. HS 19-01316), and informed consent was waived.
Data analysis was limited to primary adult-isolated ITX between 2012 and 2019. Pediatric patients (<18 years old) with two or more ITXs, and ITX with liver graft were excluded in order to form a relatively homogeneous study group. All patients received an ascending colon, in addition to the entire small intestine. In all patients, the clinical management strictly adhered to the protocol below protocol. The data entry was closed on December 31, 2019.
LABScreen SBA (One Lambda Inc., Canoga Park, CA, USA) was used to generate a profile of anti-HLA Abs (reported in a table with MFI units for each HLA) in all patients before ITX and at the time of ITX. We attempted to avoid donors at the organ offer stage when the recipient had DSA with a MFI of ≥10,000. However, this limit on organ acceptance was not absolute, and in some cases, ITX proceeded based on the recipient’s clinical condition despite the presence of DSA. vXM was performed for study purposes with various MFI cutoffs (500, 1,000, 2,000, 5,000, and 10,000), and the results were recorded in the database in real time. The results of vXM did not influence organ acceptance. pXM was always performed using two methods: flow cytometry and complement-dependent cytotoxicity (CDC) tests with recipient serum on the day of ITX and donor lymphocytes extracted from lymph nodes. SBA, which allowed the performance of vXM, was repeated with serum from the day of ITX in addition to pXM.
Anti-thymocyte immunoglobulin was used for induction, starting intraoperatively at 2 mg/kg, with two further daily doses to a total dose of 6 mg/kg. High-dose steroid induction was also used alongside and was rapidly tapered. In addition to this routine induction immunosuppressive therapy, patients with positive pXM were treated with plasma exchange, intravenous immunoglobulin, and B-lymphocyte depleting agent, starting immediately after pXM. Maintenance immunosuppression comprised tacrolimus and steroids. The target level of tacrolimus was 15 ng/dL, which slowly decreased to 10 ng/dL or lower at 1 year after ITX. Steroids were usually tapered off 1 year after ITX. Sirolimus was added for nephron sparing in selected patients who developed tacrolimus-related renal insufficiency after ITX.
Strict criteria were used to diagnose AR. There was no empirical diagnosis and/or treatment for AR. All AR presented with clinical symptoms or signs, compelling endoscopic findings, and definite histologic features. When the diagnosis was unclear, endoscopy and biopsy were repeated without altering immunosuppression until a confirmatory diagnosis was reached. Mild to moderate AR episodes were treated with a steroid bolus. Anti-thymocyte immunoglobulin was used only for severe or steroid-resistant AR with 1–2 mg/kg daily to a total dose of 6–10 mg/kg based on the clinical progress of AR.
The results of vXM with serum on the day of ITX and CDC pXM were used for statistical analyses. Pearson’s chi-square test or Fisher’s exact test was used to compare categorical variables. Kaplan-Meier survival analysis was performed to graphically represent the risk of progression to AR. Log-rank test was used to evaluate the equality of progression curves from the Kaplan-Meier analysis. All analyses were performed using the IBM SPSS ver. 24.0 (IBM Corp., Armonk, NY, USA). Statistical significance was set at P<0.05.
Fifty-six consecutive primary adult ITXs were isolated during the study period. Eight patients underwent simultaneous kidney transplantation, and three received pancreas as an en bloc organ in addition to intestinal allograft. The mean ages (±standard deviation) of the recipients and donors were 43.8 (±13.1) and 9.9 (±7.6) years old, respectively. The mean weight ratio between donor and recipient was 47.4%, with the lowest being 20.3% and the highest being 76.9%. Twelve ITXs were performed between ABO-compatible donors and recipients, and the remainder were ABO-identical. Twenty-six patients were cytomegalovirus-naïve, 14 of whom (25%, 14/56) received an organ from a cytomegalovirus-positive donor. The leading causes of ITX were mesenteric vascular incident (n=15), Crohn disease (n=13), dysmotility syndrome (n=9), trauma (n=6), surgical failure after various abdominal operations (n=6), familial adenomatous polyposis accompanied by desmoid tumor (n=4), and others (n=3). The most common indication for ITX was repeated life-threatening line sepsis (n=34, 61%). The mean cold ischemia duration was 7 hours 13 minutes, with the shortest duration being 4 hours 22 minutes and the longest being 10 hours 26 minutes. Nineteen ITXs (34%) were performed without a stoma. Six patients (11%) had ITX with a positive CDC pXM.
Twenty-eight ARs occurred in 20 patients during the study period. Six patients had one or more ARs, among whom, three patients were CDC pXM-positive. Eighteen ARs (64%) occurred during the first year after ITX, and the 1-year AR free survival rate was 67.1%. Fifteen severe ARs occurred in 12 patients, which led to eight graft losses (53%, 8/15). Mild and moderate AR responded well to the treatment, and all grafts were saved. AR was the second most common cause of graft loss following graft loss due to patient death with a functioning graft (n=11). All six patients with positive CDC pXM eventually developed nine individual episodes of AR after ITX, five patients after less than a year, and one patient at 4.3 years after ITX. Four severe ARs were noted, all of which led to graft loss in patients with positive CDC pXM. Positive CDC pXM was strongly associated with the development of AR (P<0.001), severe AR (P=0.017), and graft loss due to AR (P=0.001) using the Kaplan-Meier survival log-rank test.
There was a considerable discrepancy between the results of vXM and pXM, regardless of the MFI cutoff (Table 1). The number of positive vXMs decreased with increasing MFI cutoff. The most effective and meaningful cutoff, which provided the best association between vXM and pXM among the tested cutoffs, was 2,000. The number of positive vXM with an MFI cutoff of 2,000 was 11, with a sensitivity of 83% and specificity of 88%. Of note, the false positive rate was 54%, with an MFI cutoff of 2,000.
Among 11 positive vXMs, six patients (55%) experienced eight episodes of AR: three mild, two moderate, and three severe. All three severe ARs resulted in graft loss. The result of vXM did not correlate with development of AR (P=0.306), severe AR (P=0.885), or graft loss due to AR (P=0.434) by Kaplan-Meier survival log-rank test.
The number of DSA and MFI values for each HLA in cases of positive vXM (n=11) are shown in Table 2 along with the pXM results. Six positive vXM patients (55%) had negative pXM (patients 6–11 in Table 2). DSA to HLA Class I or II was randomly distributed and was not associated with the result of pXM. There was a trend to suggest that more DSA and higher MFI values resulted in a positive pXM; the summation of the MFI values of each DSA (ƩMFI) represents this trend. Our approach was to create a “sum MFI threshold” point; this was done by computing a summed continuous MFI score for all DSAs >2,000 MFI, defined as: x1 I(x1>2,000)+x2 I(x2>2,000)+...+xk I(xk>2,000), where "I(xk>2,000)" is an "indicator function" and equal to “1” if the condition is true (i.e., if xk>2,000) and zero otherwise. Examination of the different ƩMFI thresholds suggested that positive vXM with an MFI ≥5,000 may provide a practical guideline correlating to pXM.
Positive vXM with an MFI ≥5,000 demonstrated a good association with pXM, with sensitivity and specificity of 83% and 98%, respectively (P<0.001) (Table 3). The false positive rate of vXM decreased from 54% to 17% with enhanced vXM. Six patients met the enhanced criteria. Five patients developed positive CDC pXM at the time of ITX, all of whom eventually experienced early or late-onset AR. One patient who had a negative CDC pXM did not develop AR for 6.5 years after ITX and showed excellent graft function. Seven episodes of AR were observed in these six patients, three of which were severe. Of the three severe ARs, all resulted in graft loss; one developed less than a year and the others developed 1.7 and 4.3 years after ITX. Using the enhanced vXM showed a correlation with development of AR (P=0.013) and graft loss due to AR (P=0.047) by Kaplan–Meier log-rank test. However, we could not demonstrate a significant association with the occurrence of severe AR (P=0.267). Using ƩMFI with an enhanced vXM as the basis for a proposed guideline, the pXM and outcome of ITX are summarized in Fig. 1 by time sequence from left to right.
Numerous valuable reports have demonstrated that the presence of DSA, whether present before or after ITX, is associated with more frequent and serious AR and eventually results in poor graft survival [3-9]. The majority of ITX centers perform anti-HLA Ab tests at the time of listing, vXM when organ offer is available, and pXM at the time of ITX. In addition to this standard practice, some centers follow DSA after ITX as part of a protocol or when AR occurs. The purpose of both vXM and pXM is to detect preformed DSAs. Theoretically, the presence of preformed DSA, identified via a positive vXM, should result in a positive pXM. Therefore, a high rate of false positives in the vXM will result in prolonged time on the waiting list and effectively penalize patients for “presumed” immunological risk. Abu-Elmagd et al. [5] reported a strong association between DSA, either preformed or de novo, and long-term intestinal allograft survival using an MFI cutoff of 1,000. Data from this report demonstrated that preformed DSA, identified through a vXM, was poorly predictive of the CDC pXM result. Among 49 patients with positive vXM, 10 (20%) had negative CDC pXM, and 6% of patients with negative vXM ultimately had positive CDC pXM. Data from Cheng et al. [8] also demonstrated a discrepancy between vXM and CDC pXM in their report investigating prevalence and role of DSA on ITX outcome. In this study of 109 patients, of 12 patients with positive vXM using an MFI cutoff value of 1,000 only two (17%) had a positive CDC pXM. Moreover, 7% of patients (5/73) with negative vXM developed positive CDC pXM. Therefore, vXM, as currently applied, does not seem to be an adequate substitute for pXM and should be optimized for use in clinical practice as an effective predictor of pXM. The limited value of vXM and its relatively poor ability to predict pXM drove our current attempt to develop a guideline that can be applied more reliably in the virtual donor and recipient matching process.
There are several unique features of our study, which makes our findings valid and reliable. First, the study group was homogeneous. We designed the selection criteria to exclude possible confounding factors affecting immunologic responses and the development of AR as much as possible. As such, the study group was limited to adults with primary isolated ITX. Patients who had two or more ITXs were also excluded to avoid introducing bias from within-subject correlation. The inclusion of subsequent transplants within our survival analysis would treat each ITX as an independent event, and therefore introduce bias by ignoring the assumption that recurrent events are correlated [10,11]. Because of the potential impact of liver grafts on additional immune protection and the lower risk of AR, the study group was limited to isolated ITX [5,9]. These stricter inclusion criteria reduced the total number of patients available for analysis, but we believe that they served to limit potential confounding factors. Second, all data in this study were obtained from a prospectively maintained database. Pre-ITX follow-up, organ acceptance, transplant surgery, and post-ITX management strictly adhered to the clinical protocol, and all data were recorded prospectively. The anti-HLA Ab test was performed at a single laboratory facility using the same technique under strict quality control. The immunosuppressive therapy policy was the same in all patients throughout the study period. There was no clinical diagnosis or empiric treatment for AR, and the diagnosis of AR followed strict criteria, composed of relevant symptoms and signs, and definite histologic findings. All equivocal or inconclusive diagnoses were confirmed by repeated biopsies without altering the immunosuppressive therapy. No patient was lost during follow-up. This unique feature minimizes the era effect and unconscious bias. Of note, all data pertaining to vXM were from the serum collected on the day of ITX. In clinical practice, the available vXM results are from historical serum, not current serum. In the interest of uniformity, we decided to use results using serum on the day of ITX because there is a possibility that historical serum may not reflect current Ab status. Although the data on the correlation between historical and current serum is not included in this manuscript, there was a good correlation with only minor variations. We believe that these potential differences should not affect the wider application of our observations in routine clinical practice.
Our data highlight the close relationship between positive pXM and AR, which is consistent with the published literature. It is important to emphasize that AR can occur in the absence of a positive pXM, even though a positive pXM is clearly a signal for increased risk of AR. Indeed, the six patients with positive pXM in our cohort had nine episodes of AR, four of which were severe, and all of the severe AR episodes led to graft loss. It is also noteworthy that five of the nine episodes of AR were mild or moderate and were successfully treated without any impact on graft survival. Thus, the decision to accept the risk of positive pXM will be center-specific and contingent upon appetite for risk as well as recipient factors. This decision-making is beyond the scope of the current report. It is also of interest that in the context of preformed DSA, all episodes of AR in our dataset are episodes of acute cellular rejection. We have not seen a clear example of antibody-mediated rejection, which remains an uncommon event in ITX. Notwithstanding these unresolved issues, the ability to improve the correlation between a pre-ITX vXM and the pXM, in order to predict the likelihood of a positive pXM with greater confidence, appears to be of great importance in attenuating the risk of AR after ITX.
A relatively recent technique, SBA, provides an opportunity to identify preformed DSAs with ease, and more importantly, ahead of ITX. Hawksworth et al. [12] reported that vXM allowed successful ITX in their sensitized patients by immunologic risk reduction, which prompted the authors to examine the efficacy of vXM, focusing on the identification of a high-risk ITX. We attempted to identify relevant MFI thresholds to define DSA. The results of SBA, a profile of anti-HLA Ab, were translated into DSA and vXM based on the MFI cutoff. We hypothesized that a valid MFI threshold means an MFI threshold that yields a vXM, which correlates best with pXM. The most commonly used MFI cutoffs in the literature were 1,000 and 2,000. We tested MFI values of 0, 500, 1,000, 2,000, 5,000, and 10,000. As shown in the Results section, our retrospective analysis of vXM revealed variable correlations with pXM based on different MFI thresholds, with different sensitivities and specificities. If the clinician’s purpose in performing the vXM is to identify low-risk ITX, high specificity (probability of a negative test given that the condition is negative) is important. However, if the purpose is to avoid a high-risk ITX, high sensitivity (probability of a positive test given that the condition is true) is ideal. However, vXM with high sensitivity would carry too many false positives and therefore risk excluding too much lower risk, that is, pXM-negative ITX. Identifying sweet spots of sensitivity and specificity is critical for identifying meaningful MFI thresholds. The universal application of our conclusion, an MFI threshold of 2,000 to identify preformed DSAs, with an effective association between vXM and pXM, is debatable. Valid and useful associations depend on additional factors, including the organ acceptance policy, immunosuppression, the graft surveillance policy, and the diagnostic criteria of AR. The MFI threshold of 2,000 was the most effective in our study group in our clinical practice. With this caveat, each center may determine different thresholds, with a yield of DSA and vXM correlating the best to pXM. Without such an examination, the valid MFI cutoff in each center remains unknown. Despite using the most valid MFI cutoff to identify preformed DSAs, indicating a good association with pXM, our own false positive rate of 54% was unacceptably high. Furthermore, unlike pXM, positive vXM was unrelated to the development of AR, severe AR, or graft loss due to AR. Therefore, it became clear that a positive vXM was inadequate to abort a potential ITX, and we needed guidance that is more meaningful. Our data demonstrated that DSA with a low MFI did not affect the pXM result. On the contrary, the number of DSAs and their MFI values seemed more closely related to pXM. To develop a guideline including this concept, we adapted ƩMFI to create a new guideline. We should emphasize that ƩMFI >5,000 is arbitrary and should not be viewed as an absolute number. Indeed, our results were the same when we used any value above 5,000 for ƩMFI in our data.
In an ongoing effort to avoid positive pXM in ITX, we propose enhancements to the current vXM, which predicts pXM better than the simple vXM in its current form. The refined guideline showed better association with pXM and was associated more closely with outcomes of ITX, such as the development of AR and graft loss due to AR. Therefore, we believe this guideline is a valid substitute for the unrefined vXM in its current form and is more effective at predicting a positive pXM. In the current era, when there are no effective treatments for severe AR, avoiding high-risk ITX is an important strategy to improve the outcomes of ITX. However, decreasing the number of erroneously and unnecessarily aborted ITX due to presumed potential immunological risk from the current limitations of vXM is equally important in this life-saving procedure. We make no recommendations on the ultimate determination of risk in proceeding with an ITX, which is influenced by many factors, especially the recipient clinical status and center experience. In the absence of data, sound judgment must be ruled out. We view our refined guideline as a risk assessment strategy that attenuates the risk of AR development without avoiding ITX, which is consistent with our program philosophy. We perform endoscopy and biopsy only for cause and do not create ostomy for the purpose of frequent endoscopic evaluation [13,14]. We hope that this guideline provides the ability to identify high-risk patients and to plan an individualized strategy for graft surveillance and immunosuppressive therapy.
In conclusion, pXM is a strong risk factor for the development of AR and graft survival. vXM with a MFI of 2,000 showed the best association with pXM. However, vXM resulted in a high false positive rate, and a positive vXM was not a risk factor for the development of AR or poor graft survival. vXM enhanced by ƩMFI greater than 5,000, representing the number and strength of DSA, demonstrated better association with pXM, which in turn correlates with the development of AR and graft survival.
ACKNOWLEDGMENTS
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: JIM. Data curation: JIM. Formal analysis: HMK. Methodology: JIM. Project administration: JIM, KRI. Visualization: JIM, KRI. Writing–original draft: JIM. Writing–review & editing: KRI.
REFERENCES
1. Bond G, Reyes J, Mazariegos G, Wu T, Schaefer N, Demetris J, et al. 2000; The impact of positive T-cell lymphocytotoxic crossmatch on intestinal allograft rejection and survival. Transplant Proc. 32:1197–8. DOI: 10.1016/S0041-1345(00)01181-7. PMID: 10995904. PMCID: PMC3005342.

2. Abu-Elmagd KM, Costa G, Bond GJ, Soltys K, Sindhi R, Wu T, et al. 2009; Five hundred intestinal and multivisceral transplantations at a single center: major advances with new challenges. Ann Surg. 250:567–81. DOI: 10.1097/SLA.0b013e3181b67725. PMID: 19730240.
3. Farmer DG, Venick RS, Colangelo J, Esmailian Y, Yersiz H, Duffy JP, et al. 2010; Pretransplant predictors of survival after intestinal transplantation: analysis of a single-center experience of more than 100 transplants. Transplantation. 90:1574–80. DOI: 10.1097/TP.0b013e31820000a1. PMID: 21107306.

4. Tsai HL, Island ER, Chang JW, Gonzalez-Pinto I, Tryphonopoulos P, Nishida S, et al. 2011; Association between donor-specific antibodies and acute rejection and resolution in small bowel and multivisceral transplantation. Transplantation. 92:709–15. DOI: 10.1097/TP.0b013e318229f752. PMID: 21804443.

5. Abu-Elmagd KM, Wu G, Costa G, Lunz J, Martin L, Koritsky DA, et al. 2012; Preformed and de novo donor specific antibodies in visceral transplantation: long-term outcome with special reference to the liver. Am J Transplant. 12:3047–60. DOI: 10.1111/j.1600-6143.2012.04237.x. PMID: 22947059.
6. Gerlach UA, Lachmann N, Sawitzki B, Arsenic R, Neuhaus P, Schoenemann C, et al. 2014; Clinical relevance of the de novo production of anti-HLA antibodies following intestinal and multivisceral transplantation. Transpl Int. 27:280–9. DOI: 10.1111/tri.12250. PMID: 24279605.
7. Kubal C, Mangus R, Saxena R, Lobashevsky A, Higgins N, Fridell J, et al. 2015; Prospective monitoring of donor-specific anti-HLA antibodies after intestine/multivisceral transplantation: significance of de novo antibodies. Transplantation. 99:e49–56. DOI: 10.1097/TP.0000000000000614. PMID: 25769071.
8. Cheng EY, Everly MJ, Kaneku H, Banuelos N, Wozniak LJ, Venick RS, et al. 2017; Prevalence and clinical impact of donor-specific alloantibody among intestinal transplant recipients. Transplantation. 101:873–82. DOI: 10.1097/TP.0000000000001391. PMID: 27490417. PMCID: PMC7228620.

9. Talayero P, Ramos Boluda E, Gómez Massa E, Castro Panete MJ, Prieto Bozano G, Hernández Oliveros F, et al. 2018; Donor-specific antibodies in pediatric intestinal and multivisceral transplantation: the role of liver and human leukocyte antigen mismatching. Liver Transpl. 24:1726–35. DOI: 10.1002/lt.25323. PMID: 30112820.

10. Thenmozhi M, Jeyaseelan V, Jeyaseelan L, Isaac R, Vedantam R. 2019; Survival analysis in longitudinal studies for recurrent events: applications and challenges. Clin Epidemiol Glob Health. 7:253–60. DOI: 10.1016/j.cegh.2019.01.013.

11. Twisk JW, Smidt N, de Vente W. 2005; Applied analysis of recurrent events: a practical overview. J Epidemiol Community Health. 59:706–10. DOI: 10.1136/jech.2004.030759. PMID: 16020650. PMCID: PMC1733116.

12. Hawksworth JS, Rosen-Bronson S, Island E, Girlanda R, Guerra JF, Valdiconza C, Matsumoto CS, et al. 2012; Successful isolated intestinal transplantation in sensitized recipients with the use of virtual crossmatching. Am J Transplant. 12 Suppl 4:S33–42. DOI: 10.1111/j.1600-6143.2012.04238.x. PMID: 22947089.

13. Moon JI, Schiano TD, Iyer KR. 2019; Routine surveillance endoscopy and biopsy after isolated intestinal transplantation-revisiting the gold standard. Clin Transplant. 33:e13684. DOI: 10.1111/ctr.13684. PMID: 31374126.

14. Moon JI, Zhang H, Waldron L, Iyer KR. 2020; "Stoma or no stoma": first report of intestinal transplantation without stoma. Am J Transplant. 20:3550–7. DOI: 10.1111/ajt.16065. PMID: 32431016.

Fig. 1
Post-hoc correlation between proposed guideline using enhanced virtual cross match (vXM) with summation of the mean fluorescence intensity values of each donor-specific antibody (ƩMFI), physical cross-match (pXM), and outcome of intestinal transplantation (ITX). AR, acute rejection; CDC, complement-dependent cytotoxicity; MFI, mean fluorescence intensity. a)Positive xVM with one donor-specific anti-human leukocyte antigen antibody to DR11 (MFI, 14,382) but resulted negative pXM. No episode of AR with good graft function on 6.6 years follow-up; b)Strong auto-Ab due to Lupus. Negative vXM but resulted positive pXM. Developed mild AR 5 month and severe AR 1.5 year after ITX due to non-compliance.
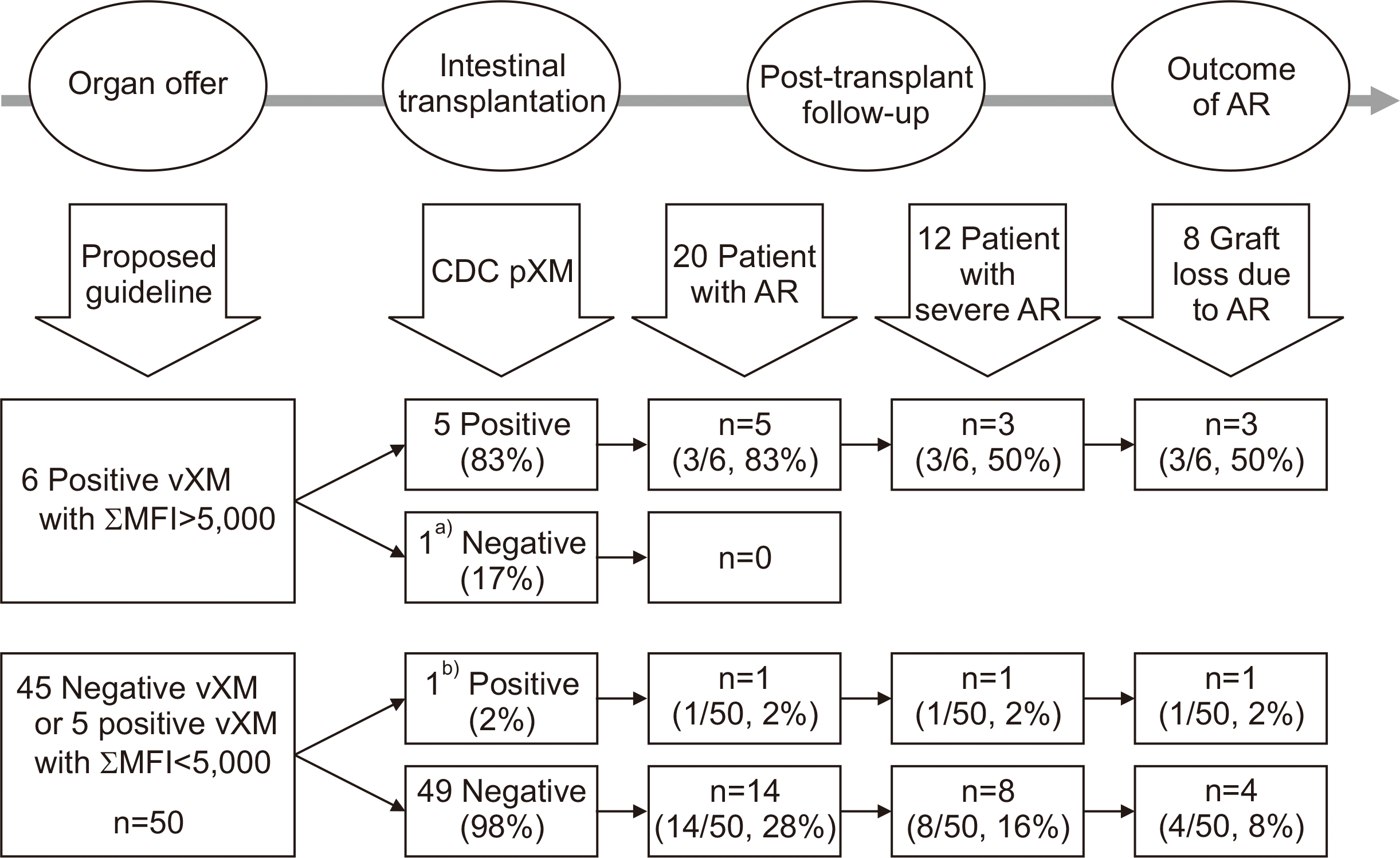
Table 1
Correlation between vXM and pXM with MFI cutoffs established at different levels
Table 2
Number of DSA and distribution of MFI to each HLA in positive vXM patients
| Patient | No. of DSAs | ƩMFI | HLA class I | HLA class II | CDC pXM | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||||||
| A | B | C | DR | DR51 | DR52 | DR53 | DQ | T cell | B cell | ||||||||
| 1 | 7 | 16,331 | 9,448 | 181 | 5,959 | 43 | 106 | 47 | 4,430 | 2,527 | 4,178 | 2,343 | 2,853 | Pos | Pos | ||
| 2 | 4 | 61,602 | 148 | 24,265 | 1,024 | 11,809 | 19,791 | 5,737 | 658 | 629 | 112 | 114 | 124 | Pos | Pos | ||
| 3 | 4 | 28,567 | 10,488 | 8,621 | 4,871 | 4,587 | 185 | 0 | 0 | 656 | 182 | 20 | 9 | Pos | Pos | ||
| 4 | 3 | 10,045 | 0 | 44 | 0 | 18 | 640 | 378 | 3,210 | 231 | 841 | 4,363 | 2,472 | Pos | Pos | ||
| 5 | 1 | 23,339 | 1,364 | 1,099 | 649 | 23,339 | 195 | 271 | 596 | 926 | 300 | Pos | Pos | ||||
| 6 | 1 | 14,382 | 148 | 71 | 257 | 248 | 342 | 1,014 | 11 | 14,382 | 21 | 24 | 0 | Neg | Neg | ||
| 7 | 1 | 4,576 | 128 | 1,317 | 84 | 247 | 1,464 | 136 | 342 | 642 | 505 | 4,576 | 565 | Neg | Neg | ||
| 8 | 1 | 2,485 | 9 | 70 | 237 | 618 | 371 | 124 | 1,111 | 2,485 | 773 | 379 | 120 | 87 | Neg | Neg | |
| 9 | 1 | 2,445 | 38 | 76 | 221 | 209 | 180 | 193 | 2,445 | 1,231 | 239 | 134 | 134 | Neg | Neg | ||
| 10 | 1 | 2,233 | 75 | 2,233 | 235 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1,285 | Neg | Neg | ||
| 11 | 1 | 2,201 | 126 | 430 | 1,234 | 1,943 | 257 | 403 | 352 | 2,201 | 284 | 383 | Neg | Neg | |||
| 12a) | 0 | 0 | 295 | 131 | 34 | 0 | 309 | 0 | 0 | 63 | 244 | 244 | 124 | Pos | Pos | ||
DSA, donor-specific antibody; MFI, mean fluorescence intensity; HLA, human leukocyte antigen; vXM, virtual cross match; ƩMFI, summation of the mean fluorescence intensity values of each donor-specific antibody; CDC, complement-dependent cytotoxicity; pXM, physical cross-match; Pos, positive; Neg, negative; AR, acute rejection.
Table 3
Association between enhanced vXM and pXM
| Variable | CDC pXM | |
|---|---|---|
|
|
||
| Positive (n=6) | Negative (n=50) | |
| Positive vXM with ƩMFI ≥5,000 (n=6) | 5 (83) | 1 (17) |
| Negative vXM and positive vXM with ƩMFI <5,000 (n=50)a) | 1 (2) | 49 (98) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download