Abstract
Tissue regeneration is one of the ultimate goals of maxillofacial surgery and various types of tissue engineering technologies have been utilized in clinics. Healthy resources of host cells and growth factors are essential for the tissue engineering, therefore autologous blood-derived cell therapy was introduced. In this article, clinical applications of the autologous platelet concentrates and stem cell separation therapy will be summarized and evaluated for their efficacy and feasibility in the current maxillofacial clinics.
Bone graft in maxillofacial field is a procedure performed to increase the amount of alveolar or basal jaw bones where they have been lost by diseases, trauma, or surgeries. Because of the difficulties to regenerate the bony defects completely and rapidly, various types of tissue engineering technologies have been applied in maxillofacial clinics1-4. For example, bone graft materials such as allogenic, xenogeneic, and synthetic bones were introduced and used as scaffolds, as well as barrier membranes and titanium mesh are available to facilitate proper three-dimensional (3D) shapes of bone formation. Importantly, one of the missing aspects in this endeavor is lack of healthy resources of host cells and growth factors, such as local mesenchymal stem cells or bone marrow progenitor cells with autologous growth factors. To overcome this limitation, autologous blood-derived cell therapy was introduced several decades ago consisting of platelet concentrates (PCs) such as platelet-rich plasma (PRP) and platelet-rich fibrin (PRF). In addition to the PCs, closed system of stem cell separation from the patients’ own bone marrow or blood was also developed and utilized in clinics. In this article, clinical applications of the autologous blood derived cell therapy will be summarized and evaluated for their efficacy and feasibility in maxillofacial surgery.
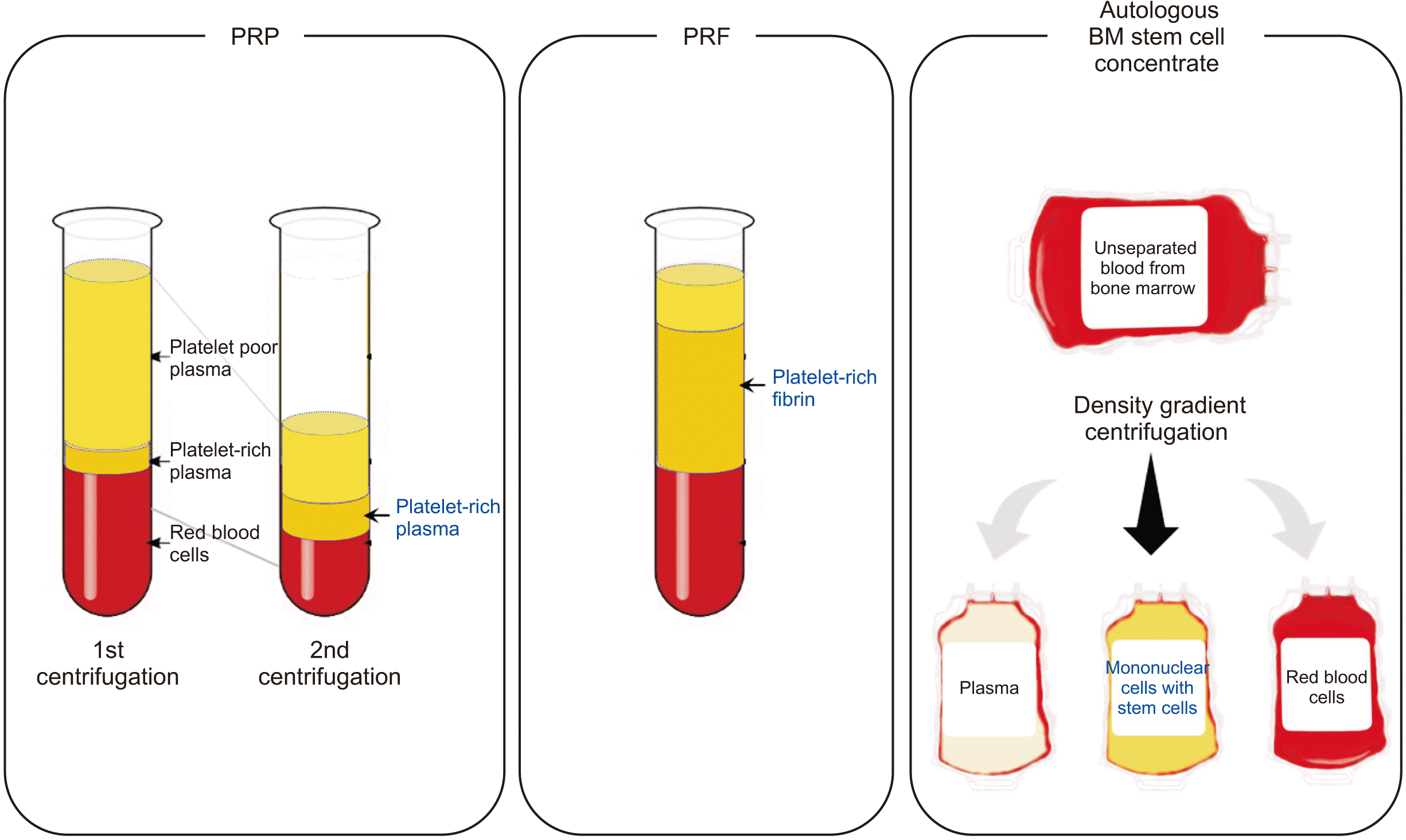
The concept of PCs was originated from the idea that platelets and autologous growth factors could be condensed in plasma and utilized to promote local wound healing5. The first generation of PCs was given the popular name PRP and is composed of over 95% platelets, a cell type that actively secretes growth factors for initiating wound healing and secreting factors responsible for enhancing cell adhesion, proliferation, and migration of various cell types6-8. However, PRP preparation requires addition of bovine thrombin or CaCl2 which makes that PRP is not 100% autologous. Furthermore, the preparation must be centrifuged in two separate stages to increase purity of platelet concentration.(Fig. 1, left) It has further been reported that the liquid nature of PRP complicates its handling by clinicians. All these limitations have led to the emergence of a second-generation PCs.
PRF can be fabricated from 100% autologous sources without any bovine thrombin or CaCl2 and can be fabricated by a one-step standardized method9-11. Through centrifugation of peripheral blood, physiologic clot formation and fractioning are induced. The natural clot formation by centrifugation requires using specific tubes with a glass surface initiates the coagulation cascade and activates platelets during centrifugation. The resulting PRF consists of a fibrin scaffold that contains platelets, leukocytes, and plasma proteins. After centrifugation, the resulted 3D fibrin matrix of the PRF serves as a reservoir of growth factors and soft tissue barriers.(Fig. 1, middle)
Efforts to separate blood cells, especially hematopoietic stem cells from patients’ own blood have been made to utilize the stem cell fractions for tissue engineering. There are a multitude of ways to sort different types of cell components and one of the quicker ways to sort a sample based on physical characteristics is density gradient centrifugation. However, the first trial to separate and transplant the autologous human bone marrow mononuclear cells was only conducted in 2001 for treatment of chronic heart disease and peripheral arterial disease using SEPAX system12. Several companies developed commercially available closed centrifugation system to efficiently separate blood cell fractions aseptically to maintain the high standards required for cellular therapeutics13-15.(Fig. 1, right) Although transplantation of the stem cell fraction is efficient for tissue regeneration, statistics and official report from the Korean Ministry of Health and Welfare in 2018 showed that only 4 autologous or allogenic stem cell products have been approved and available in clinics among all 15 cell therapy products in South Korea. However, not a single cell therapy product is approved yet for dentistry and maxillofacial surgery field.
Numerous studies on PRP and PRF were published to show efficacy for their dental tissue regeneration, such as regeneration of periodontal bony defect, tooth furcation defect, gingival defect on tooth roots, extraction socket preservation and maxillary sinus membrane elevation8-11,16. Autologous stem cells separated from blood or bone marrow also have been showed superior soft tissue wound healing in dermatology, cardiology, and plastic surgery12-15. Furthermore, recent studies showed that platelets and mesenchymal stem cells promote tissue regeneration by modulating local immune reactions17,18.
Autologous blood-derived cell products require manipulation procedures ex vivo, so that there has been contamination, safety, and standardization issues for clinical application. Additionally, health technology assessment was first introduced in South Korea in 2006, and the Committee of New Health Technology Assessment (CNHTA) was formed and started to review safety and effectiveness of the new health technology. Since then, PCs in various medical applications tried to be approved as a new medical technology by CNHTA; however, no trial was succeeded until January 2019. Currently, autologous PRP application is approved for single usage in treatment of tennis elbow; Epicondylopathia humeri radialis, and no other medical, dental, and maxillofacial usage is approved.
(1) Autologous blood-derived cell therapy can supply healthy resources of host cells and growth factors to promote tissue regeneration in maxillofacial surgery field.
(2) Although there have been controversies in efficacy and feasibility of PCs and stem cell transplantation, the autologous blood-derived cell therapy is one of the better options for regeneration of maxillofacial tissues.
(3) We need more in vivo and clinical trial data to find the proper therapeutic doses for autologous PCs and stem cells suitable for different clinical situations in maxillofacial surgery.
(4) Development of concrete and practical solutions including standardized clinical protocols, technologies and delivery systems of PCs and stem cell fractions will become essential in the future.
References
1. Ceccarelli G, Presta R, Benedetti L, Cusella De Angelis MG, Lupi SM. Rodriguez Y Baena R. 2017; Emerging perspectives in scaffold for tissue engineering in oral surgery. Stem Cells Int. 2017:4585401. https://doi.org/10.1155/2017/4585401. DOI: 10.1155/2017/4585401. PMID: 28337223. PMCID: PMC5346390.

2. Doonquah L, Holmes PJ, Ranganathan LK, Robertson H. 2021; Bone grafting for implant surgery. Oral Maxillofac Surg Clin North Am. 33:211–29. https://doi.org/10.1016/j.coms.2021.01.006. DOI: 10.1016/j.coms.2021.01.006. PMID: 33750652.

3. Rakhmatia YD, Ayukawa Y, Furuhashi A, Koyano K. 2013; Current barrier membranes: titanium mesh and other membranes for guided bone regeneration in dental applications. J Prosthodont Res. 57:3–14. https://doi.org/10.1016/j.jpor.2012.12.001. DOI: 10.1016/j.jpor.2012.12.001. PMID: 23347794.

4. Zhao R, Yang R, Cooper PR, Khurshid Z, Shavandi A, Ratnayake J. 2021; Bone grafts and substitutes in dentistry: a review of current trends and developments. Molecules. 26:3007. https://doi.org/10.3390/molecules26103007. DOI: 10.3390/molecules26103007. PMID: 34070157. PMCID: PMC8158510.

5. Masoudi E, Ribas J, Kaushik G, Leijten J, Khademhosseini A. 2016; Platelet-rich blood derivatives for stem cell-based tissue engineering and regeneration. Curr Stem Cell Rep. 2:33–42. https://doi.org/10.1007/s40778-016-0034-8. DOI: 10.1007/s40778-016-0034-8. PMID: 27047733. PMCID: PMC4817373.

6. Marx RE. 2004; Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 62:489–96. https://doi.org/10.1016/j.joms.2003.12.003. DOI: 10.1016/j.joms.2003.12.003. PMID: 15085519.

7. Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. 1998; Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 85:638–46. https://doi.org/10.1016/s1079-2104(98)90029-4. DOI: 10.1016/S1079-2104(98)90029-4.

8. Xu J, Gou L, Zhang P, Li H, Qiu S. 2020; Platelet-rich plasma and regenerative dentistry. Aust Dent J. 65:131–42. https://doi.org/10.1111/adj.12754. DOI: 10.1111/adj.12754. PMID: 32145082. PMCID: PMC7384010.

9. Miron RJ, Fujioka-Kobayashi M, Bishara M, Zhang Y, Hernandez M, Choukroun J. 2017; Platelet-rich fibrin and soft tissue wound healing: a systematic review. Tissue Eng Part B Rev. 23:83–99. https://doi.org/10.1089/ten.TEB.2016.0233. DOI: 10.1089/ten.teb.2016.0233. PMID: 27672729.

10. Miron RJ, Zucchelli G, Pikos MA, Salama M, Lee S, Guillemette V, et al. 2017; Use of platelet-rich fibrin in regenerative dentistry: a systematic review. Clin Oral Investig. 21:1913–27. https://doi.org/10.1007/s00784-017-2133-z. DOI: 10.1007/s00784-017-2133-z. PMID: 28551729.

11. Naik B, Karunakar P, Jayadev M, Marshal VR. 2013; Role of platelet rich fibrin in wound healing: a critical review. J Conserv Dent. 16:284–93. https://doi.org/10.4103/0972-0707.114344. DOI: 10.4103/0972-0707.114344. PMID: 23956527. PMCID: PMC3740636.

12. Strauer BE, Brehm M, Zeus T, Gattermann N, Hernandez A, Sorg RV, et al. 2001; [Intracoronary, human autologous stem cell transplantation for myocardial regeneration following myocardial infarction]. Dtsch Med Wochenschr. 126:932–8. German. https://doi.org/10.1055/s-2001-16579-2. DOI: 10.1055/s-2001-16579-2. PMID: 11523014.

13. Aktas M, Radke TF, Strauer BE, Wernet P, Kogler G. 2008; Separation of adult bone marrow mononuclear cells using the automated closed separation system Sepax. Cytotherapy. 10:203–11. https://doi.org/10.1080/14653240701851324. DOI: 10.1080/14653240701851324. PMID: 18368599.

14. Güven S, Karagianni M, Schwalbe M, Schreiner S, Farhadi J, Bula S, et al. 2012; Validation of an automated procedure to isolate human adipose tissue-derived cells by using the Sepax® technology. Tissue Eng Part C Methods. 18:575–82. https://doi.org/10.1089/ten.TEC.2011.0617. DOI: 10.1089/ten.tec.2011.0617. PMID: 22372873. PMCID: PMC3401386.

15. Mazzanti B, Urbani S, Dal Pozzo S, Bufano P, Ballerini L, Gelli A, et al. 2017; Fully automated, clinical-grade bone marrow processing: a single-centre experience. Blood Transfus. 15:577–84. https://doi.org/10.2450/2016.0057-16. DOI: 10.2450/2016.0057-16.

16. Kobayashi E, Flückiger L, Fujioka-Kobayashi M, Sawada K, Sculean A, Schaller B, et al. 2016; Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Investig. 20:2353–60. https://doi.org/10.1007/s00784-016-1719-1. DOI: 10.1007/s00784-016-1719-1. PMID: 26809431.

17. Cruz-Barrera M, Flórez-Zapata N, Lemus-Diaz N, Medina C, Galindo CC, González-Acero LX, et al. 2020; Integrated analysis of transcriptome and secretome from umbilical cord mesenchymal stromal cells reveal new mechanisms for the modulation of inflammation and immune activation. Front Immunol. 11:575488. https://doi.org/10.3389/fimmu.2020.575488. DOI: 10.3389/fimmu.2020.575488. PMID: 33117373. PMCID: PMC7561386.

18. Li C, Li J, Li Y, Lang S, Yougbare I, Zhu G, et al. 2012; Crosstalk between platelets and the immune system: old systems with new discoveries. Adv Hematol. 2012:384685. https://doi.org/10.1155/2012/384685. DOI: 10.1155/2012/384685. PMID: 23008717. PMCID: PMC3447344.

Fig. 1
The layers after centrifugation, the blood components and separated cells are shown among PRP (platelet-rich plasma; the most left), PRF (platelet-rich fibrin; middle) and autologous bone marrow (BM) stem cell concentrate (the most right). The final product from each preparation method is written in blue.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download