I. Introduction
II. Materials and Methods
1. Preparation of titanium disks
2. Preparation of collagen/nHAp/BMP-2 composite solution and coating
3. AFM
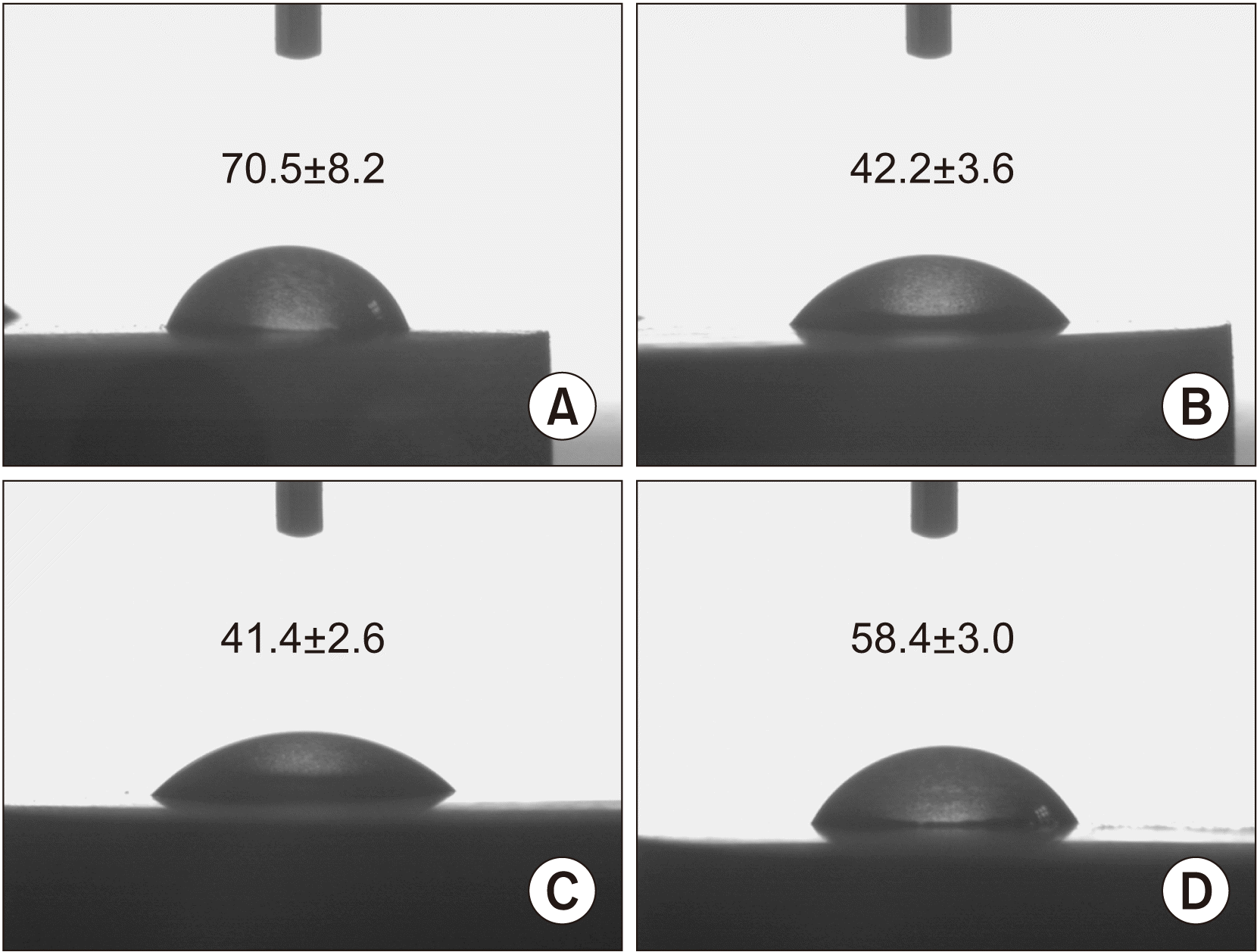
4. Contact angle
5. Release of BMP-2 from the disk
6. Bone marrow-derived mesenchymal stem cell culture
7. Flow cytometry (FACS) after differentiation
8. Biochemical assays for alkaline phosphatase (ALP), glycosaminoglycan (GAG), and osteopontin (OPN) production
9. Reverse transcription-polymerase chain reaction (RT-PCR) for BM-MSCs
Table 1
10. Mouse subcutaneous injection
11. Immunohistochemical staining
12. Implant installation on the rabbit tibia and removal torque measurement
13. Histological analysis
14. Statistical analysis
III. Results
1. Effect of nHAp and BMP-2 on titanium surface roughness and chemistry
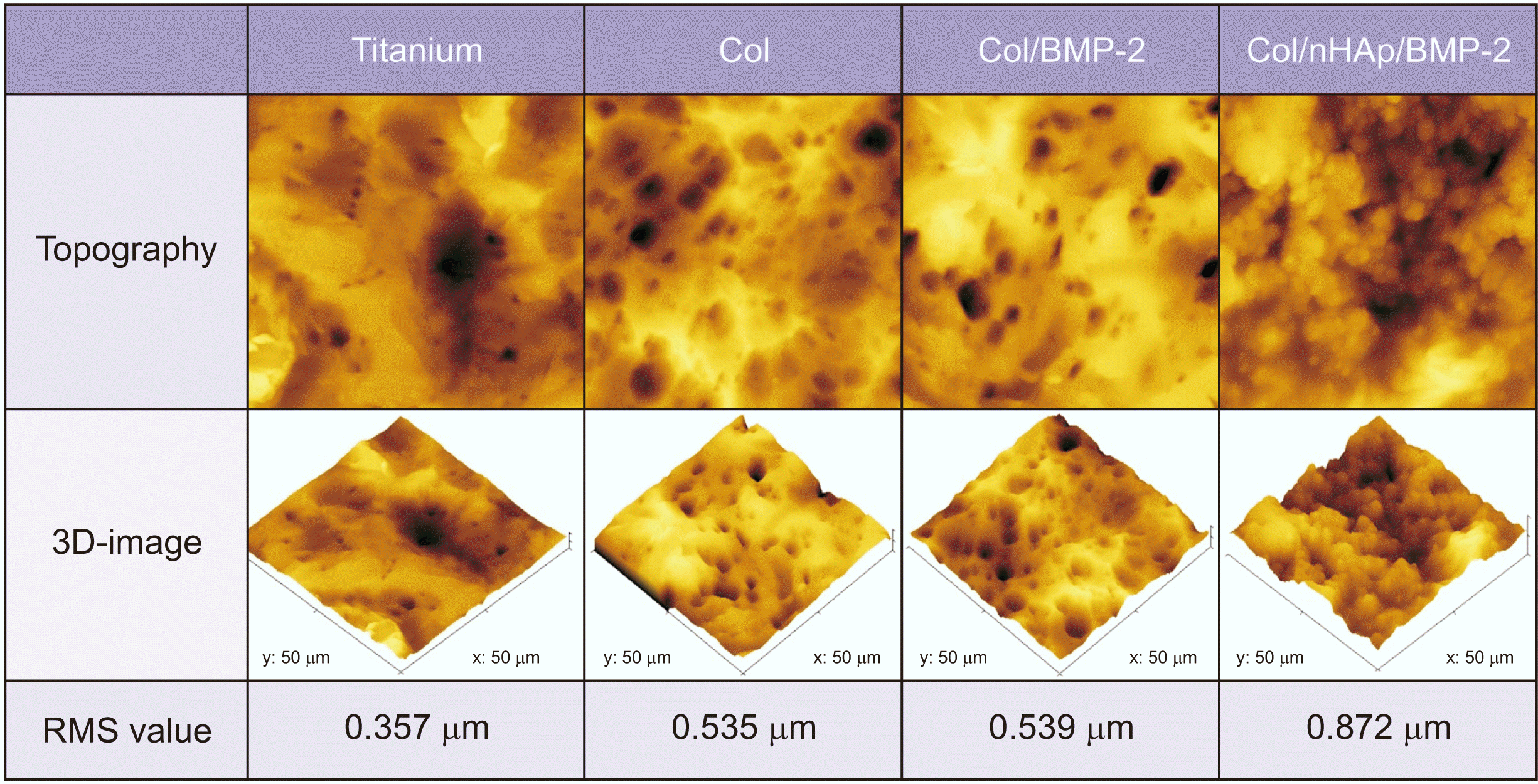 | Fig. 1Topographic and three-dimensional atomic force microscopic images of the coatings. Titanium: untreated titanium disk, Col: collagen-coated titanium surface, Col/BMP-2: collagen/bone morphogenetic protein-2 (BMP-2)-coated titanium surface, Col/nHAp/BMP-2: collagen/nano-hydroxyapatite/BMP-2-coated titanium surface. (RMS: root mean square) |
2. BMP-2 release
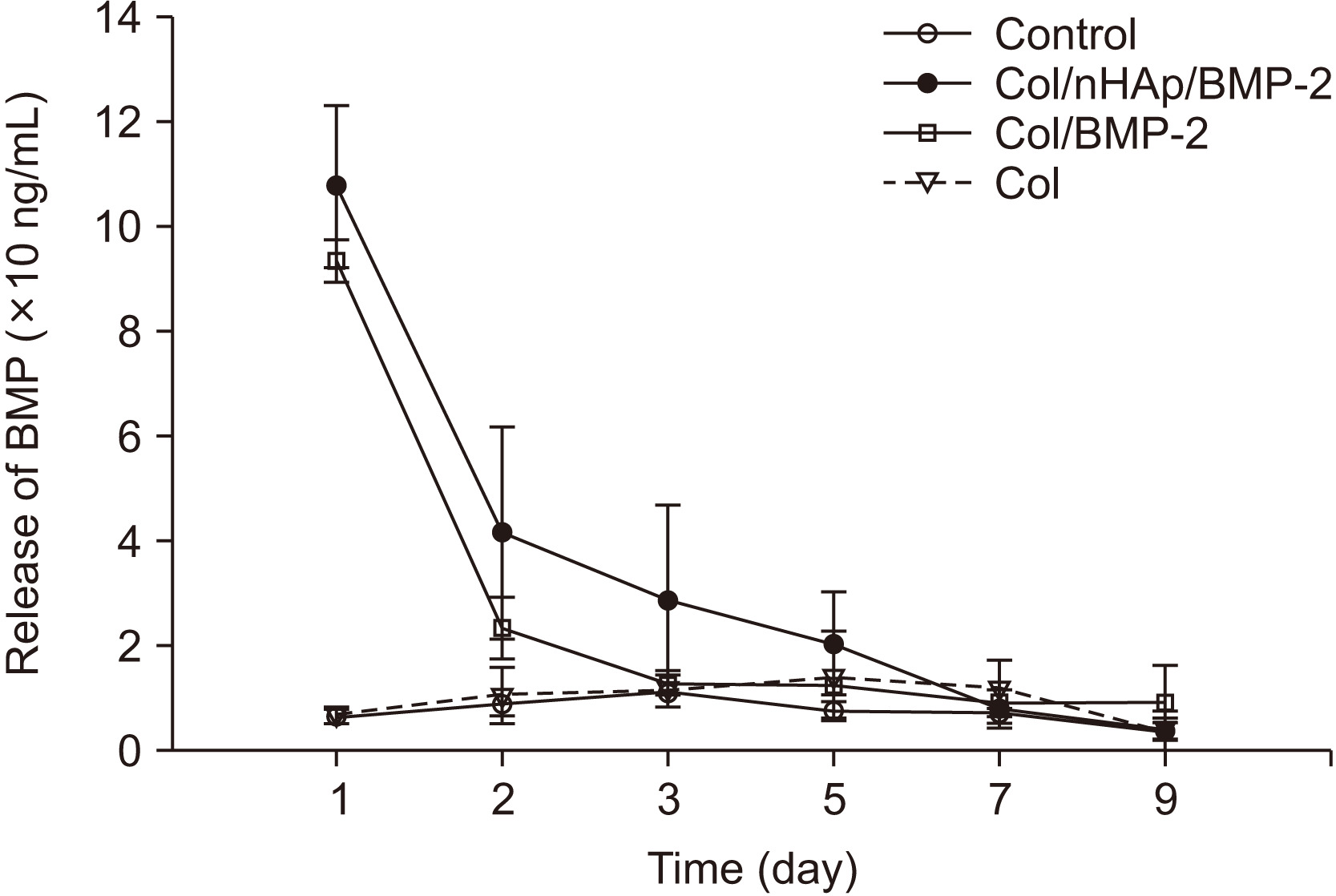 | Fig. 3
In vitro release curve of bone morphogenetic protein-2 (BMP-2) from various surface-treated titanium disks. The results are shown as mean±standard deviation values (n=3). An increase in BMP-2 release was observed within 24 hours. A sustained release in the Col/nHAp/BMP-2 group was observed between 1-5 days. (Col: collagen, nHAp: nano-hydroxyapatite) |
3. FACS analysis after differentiation
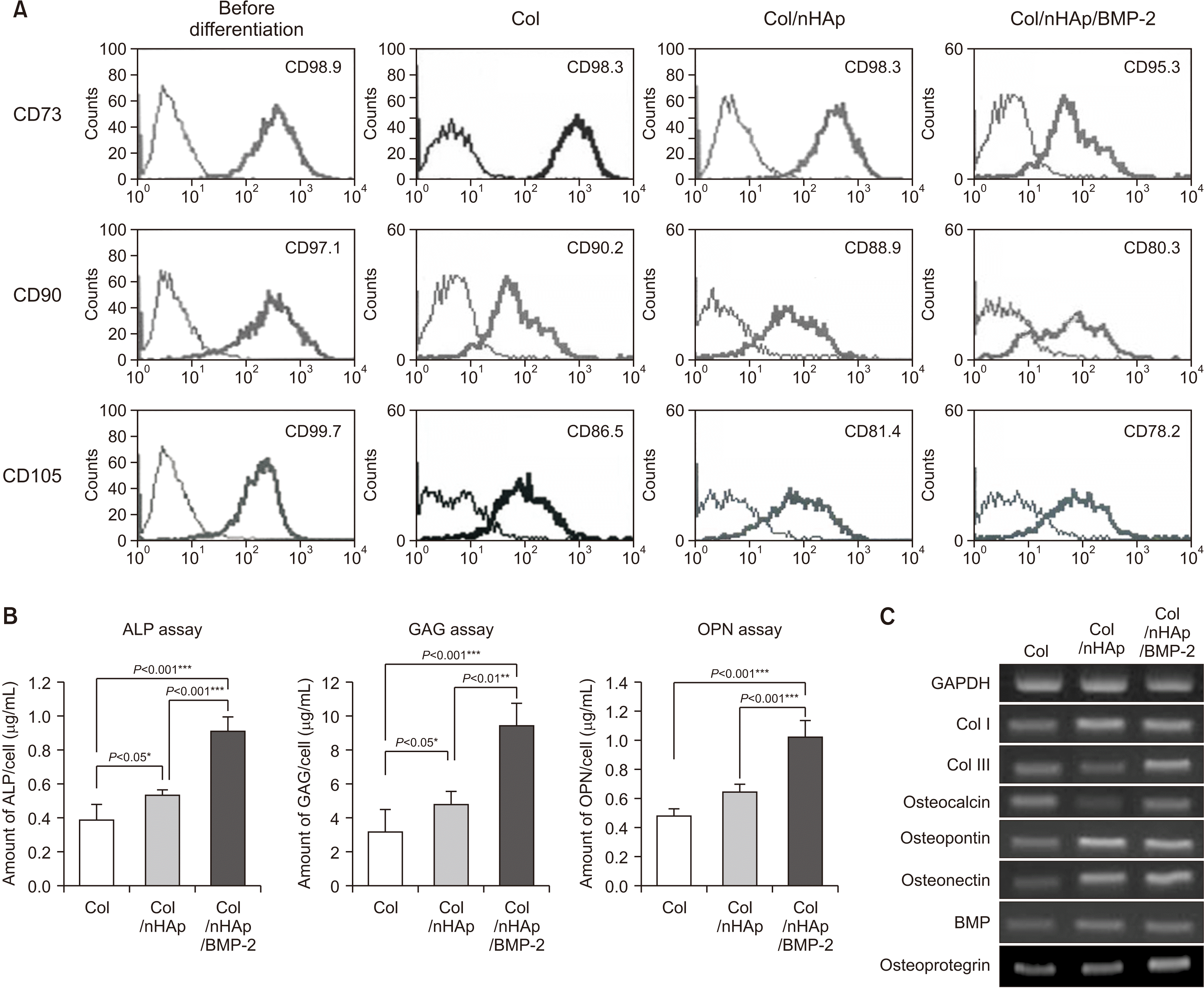 | Fig. 4Bone marrow-derived mesenchymal stem cell (BM-MSC) differentiation on differently treated titanium disks. A. Flow cytometry of typical CD markers present on BM-MSCs cultured on the Col-, Col/nHAp-, or Col/nHAp/BMP-2-coated titanium disks. CD73+, CD90+, and CD105+ cells were observed prior to differentiation. Reduction in mesenchymal CD73+, CD90+, and CD105+ cells was observed in the Col/nHAp/BMP-2 group. B. Biochemical assays for alkaline phosphatase (ALP) activity and intracellular glycosaminoglycan (GAG) and osteopontin expression in BM-MSCs. Cells on Col/nHAp/BMP-2 exhibited the highest expression. C. mRNA expression of different osteoblastic markers in BM-MSC. The highest expression was observed for type III collagen (Col III), osteocalcin, and osteoprotegerin in cells grown on Col/nHAp/BMP-2. The levels of type I collagen (Col I), osteopontin, osteonectin, and BMP-2 expression were higher in BM-MSCs on Col/nHAp and Col/nHAp/BMP-2 than in those with Col enrichment. (Col: collagen, nHAp: nano-hydroxyapatite, BMP-2: bone morphogenetic protein-2) |
4. Biochemical assays for ALP, GAG, and OPN production
5. RT-PCR
6. Mouse subcutaneous injections
 | Fig. 5Histological images of the mouse subcutaneous pocket injection model. Col (A, D, G, J, M, P), Col/nHAp (B, E, H, K, N, Q), and Col/nHAp/BMP (C, F, I, L, O, R). Macroscopic images (A-C), H&E staining (D-F), Masson’s trichrome (MT) staining (G-I), von Kossa staining (J-L), osteonectin staining (M-O), and CD31 staining (P-R) (D-O: ×100, P-R: ×200, scale bars=200 µm). (Col: collagen, nHAp: nano-hydroxyapatite, BMP-2: bone morphogenetic protein-2) |
7. Implant installation on the rabbit tibia
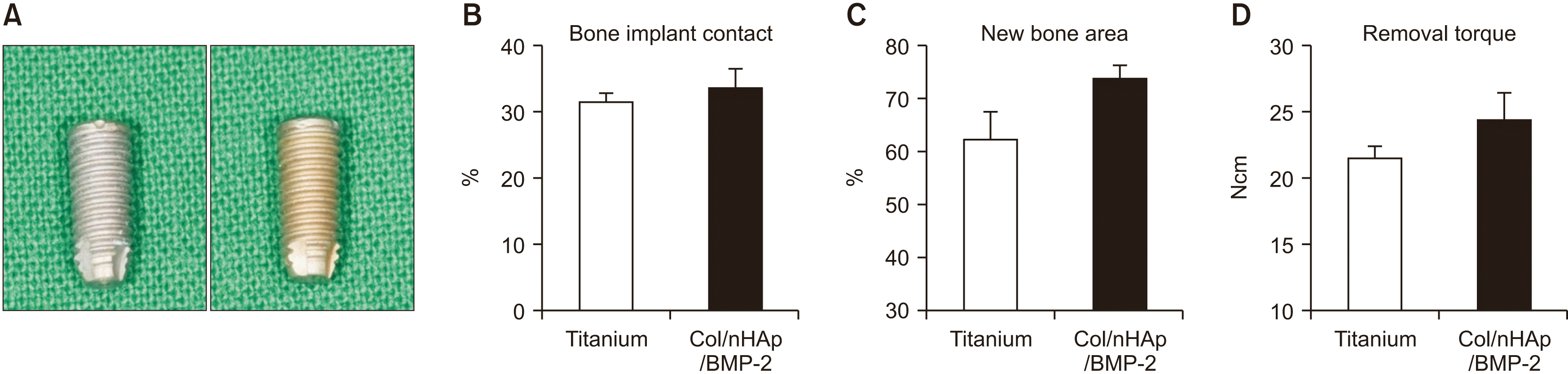 | Fig. 6Implant installation on rabbit tibia. A. Photograph of titanium implant (left) and Col/nHAp/BMP-2-coated implant (right). B-D. Bone implant contact, new bone area, and removal torque. At 4 weeks after implant installation on rabbit tibia, Col/nHAp/BMP-2 exhibited increased new bone area compared with the negative control (P=0.07), whereas bone implant contact and removal torque exhibited no significant difference, although the mean values were higher. (Col: collagen, nHAp: nano-hydroxyapatite, BMP-2: bone morphogenetic protein-2) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download