1. Singh VP, Uppoor AS, Nayak DG, Shah D. 2013; Black triangle dilemma and its management in esthetic dentistry. Dent Res J (Isfahan). 10:296–301.
3. Pitale U, Pal PC, Thakare G, Verma M, Dhakad S, Pandey R. 2021; Minimally invasive therapy for reconstruction of lost interdental papilla by using injectable hyaluronic acid filler. J Indian Soc Periodontol. 25:22–8.
https://doi.org/10.4103/jisp.jisp_19_20.

4. Beagle JR. 1992; Surgical reconstruction of the interdental papilla: case report. Int J Periodontics Restorative Dent. 12:145–51.
5. Azzi R, Etienne D, Takei H, Fenech P. 2002; Surgical thickening of the existing gingiva and reconstruction of interdental papillae around implant. Int J periodontol Rest Dent. 22:71–7.
6. Kaushik A, Pk P, Jhamb K, Chopra D, Chaurasia VR, Masamatti VS, et al. 2014; Clinical evaluation of papilla reconstruction using subepithelial connective tissue graft. J Clin Diagn Res. 8:ZC77–81.
https://doi.org/7860/JCDR/2014/9458.4881.

7. Azzi R, Takei HH, Etienne D, Carranza FA. 2001; Root coverage and papilla reconstruction using autogenous osseous and connective tissue grafts. Int J Periodontics Restorative Dent. 21:141–7.
8. Carranza N, Zogbi C. 2011; Reconstruction of the interdental papilla with an underlying subepithelial connective tissue graft: technical considerations and case reports. Int J Periodontics Restorative Dent. 31:e45–50.
10. Geurs NC, Romanos AH, Vassilopoulos PJ, Reddy MS. 2012; Efficacy of micronized acellular dermal graft for use in interproximal papillae regeneration. Int J Periodontics Restorative Dent. 32:49–58.
17. Cohen B. 1959; Pathology of the interdental tissues. Dent Pract. 9:167–73.
25. Gurinsky B. 2009; A novel dehydrated amnion allograft for use in the treatment of gingival recession: an observational case series. J Implant Adv Clin Dent. 1:65–73.
27. Joshi CP, Panjwani AA, D'Lima CB, Dani NH. 2017; Comparative evaluation of amnion-chorion membrane and chorion membrane for root coverage and gingival biotype enhancement: a case report. EC Dent Sci. 14:255–9.
28. Elzanaty M, Shoieb M, Ghallab NA, Nahass HEE. 2017; Clinical evaluation of amnion chorion membrane in comparison to subepithelial connective tissue graft in gingival recession coverage. Egypt Dent J. 30.
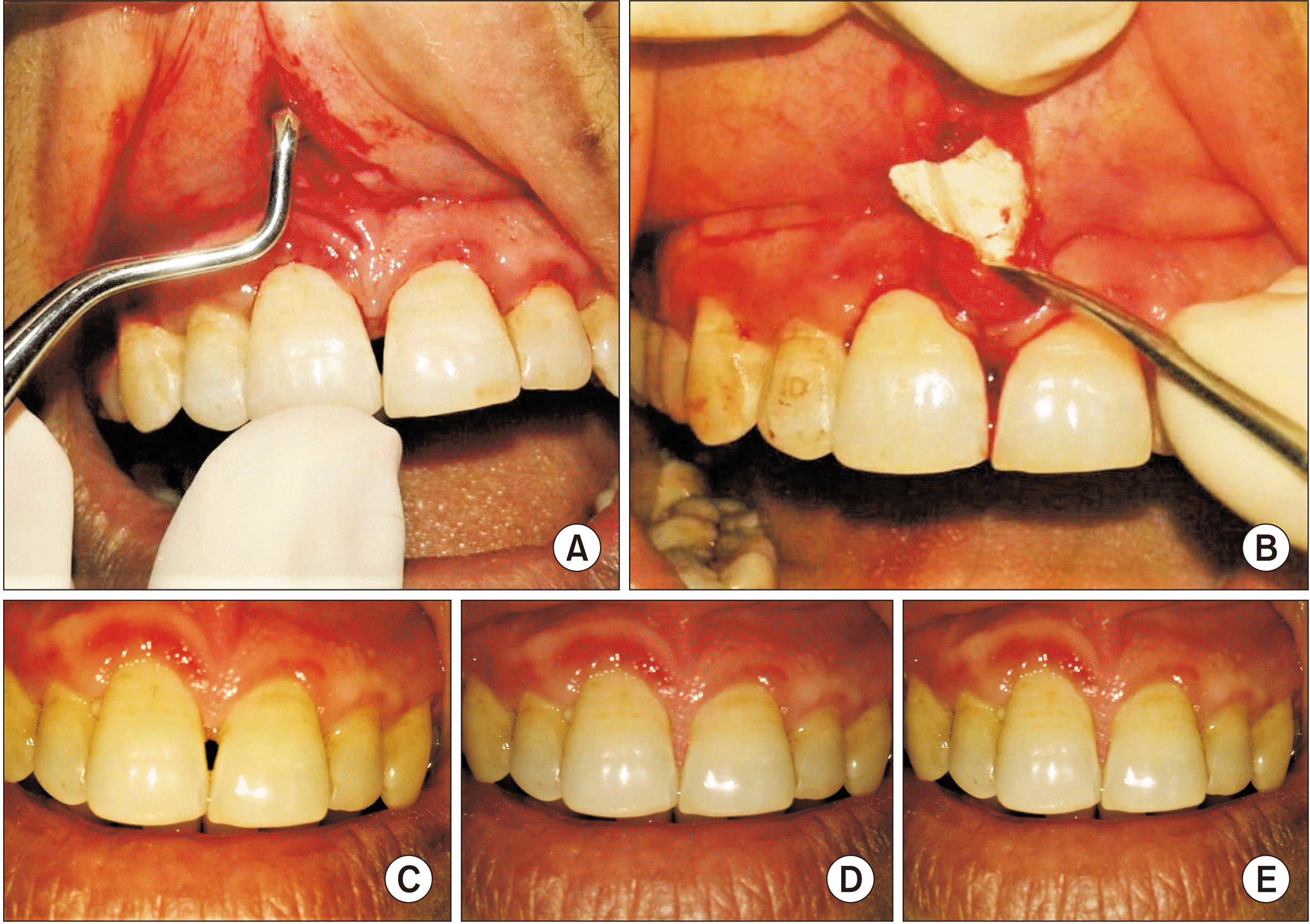
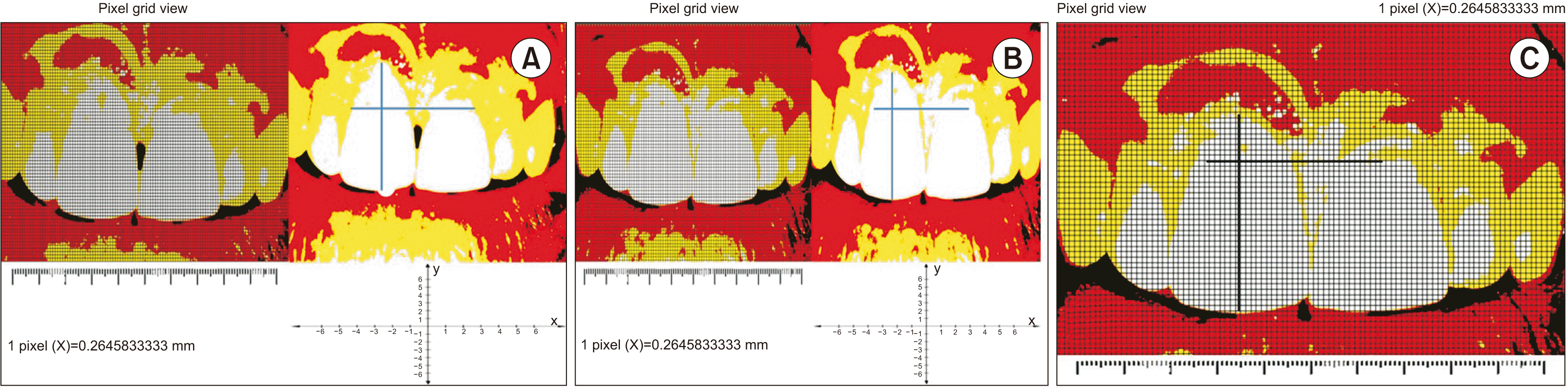




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download