Abstract
Although the JAK2 V617F mutation is a common genetic basis for the BCR-ABL1-negative myeloproliferative neoplasm (MPN), it is very rarely observed in acute myeloid leukemia (AML) without antecedent MPN. While JAK2 V617F has a well-defined role as a driver mutation in MPNs, its role in de novo AML remains elusive. Here, we retrospectively identified two patients with JAK2 V617F-positive AML by next-generation sequencing. These patients were diagnosed with AML without a history of an antecedent MPN. The presence of dysmegakaryopoiesis and a non-complex karyotype were consistent with the features reported in previous cases. Concurrent mutations in JAK2 and MPL were identified in one of the patients. Presence of the JAK2 V617F mutation in AML does not imply a blast phase of an occult MPN and suggests a separate clinical entity.
초록
JAK2 V617F는 BCR-ABL1 음성 골수증식종양(MPN)에서 높은 빈도로 발견되는 유전자 돌연변이이다. 그러나 이전 MPN 병력이 없는 급성골수성백혈병(AML)에서는 매우 드물게 관찰된다. 골수증식종양에서는 암 유발 돌연변이로서 JAK2 V617F의 역할이 잘 알려져 있지만, 일차성 AML에서 JAK2 V617F의 역할은 명확하지 않다. 본 연구에서는 후향적으로 차세대염기서열분석에 의해 두 명의 JAK2 V617F 양성 AML 환자를 찾았기에 문헌고찰과 함께 보고하는 바이다. 두 환자 모두 이전에 MPN 진단을 받지 않은 상태에서 AML 진단을 받았다. 거핵구 이형성(dysmegakaryopoiesis)과 비복합성 핵형(non-complex karyotype)이 관찰된다는 점은 이전 연구와 일치했다. 한 환자에서는 JAK2와 MPL 두 유전자에서 동시에 돌연변이가 있었는데 이는 드문 소견이었다. JAK2 V617F 양성 AML은 기저 MPN으로부터 진행하여 발생하는 이차성 AML과는 생물학적으로 구분되는 질환이다. AML 진단을 받은 환자에서 확인된 JAK2 V617F 돌연변이 양성이 반드시 기저 MPN의 급성기를 의미하지는 않으며, 별개의 질환임을 본 증례를 통해 확인할 수 있다.
The JAK2 V617F mutation is a common genetic basis for the BCR-ABL1-negative myeloproliferative neoplasm (MPN). The majority (~95%) of polycythemia vera and approximately half of the essential thrombocythemia and primary myelofibrosis patients carry this mutation [1]. However, it is rarely observed in de novo acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) [2, 3]. While JAK2 V617F has a well-defined role as a driver mutation in MPNs, its role in de novo AML remains largely unknown. We retrospectively identified two JAK2 V617F-positive AML cases and analyzed them to investigate the clinical manifestations and genetic characteristics, as well as morphological features of the bone marrow (BM) from patients carrying this mutation. This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (B1711/435-004) and informed consent was obtained from the patients.
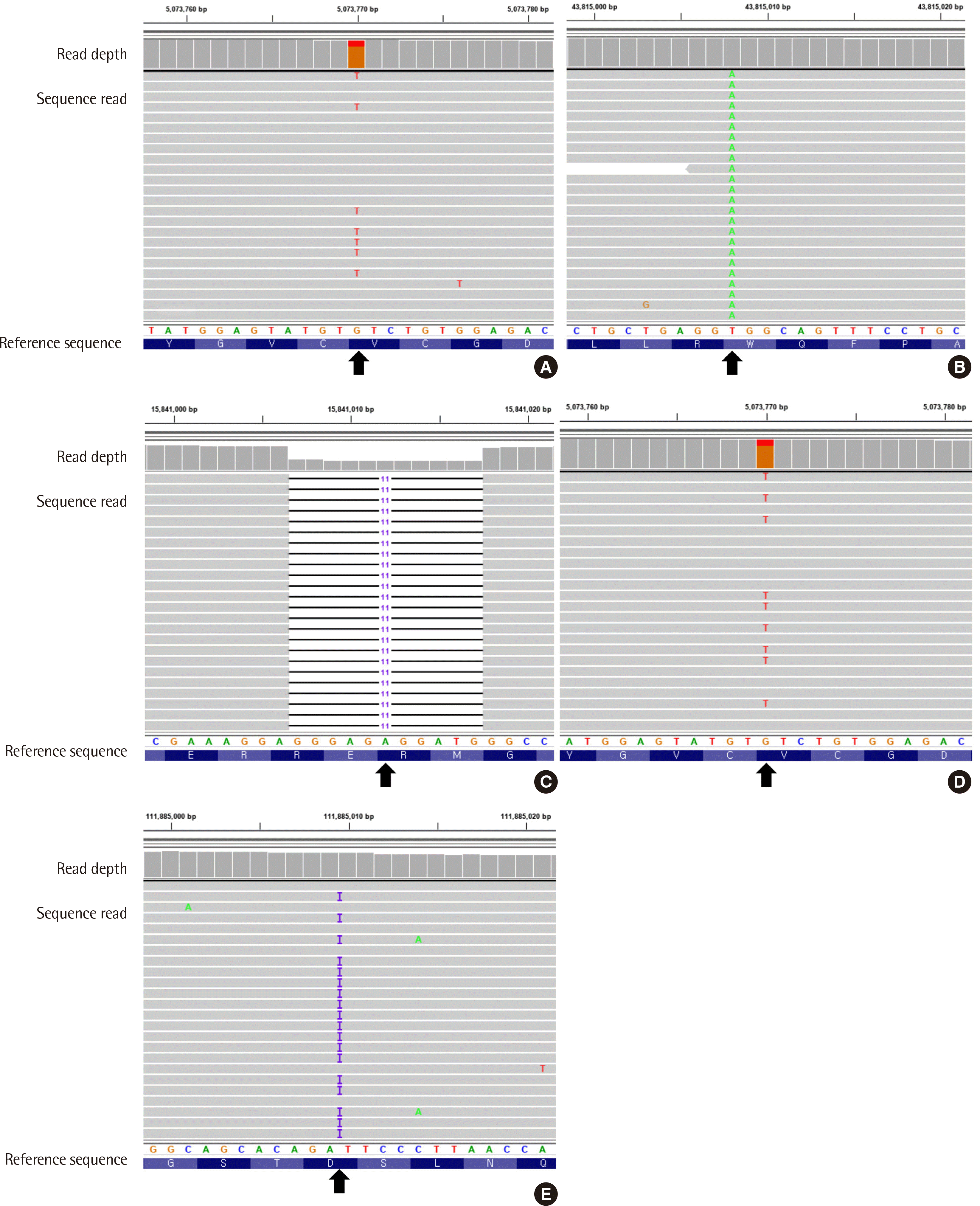
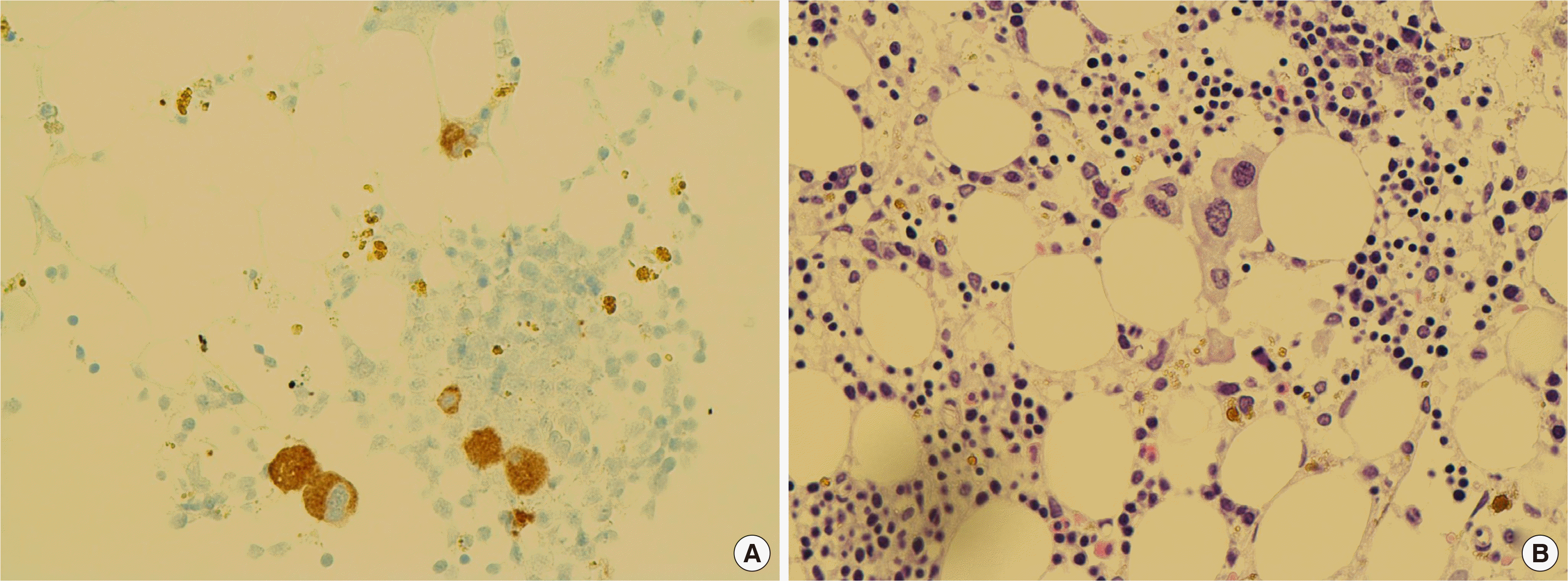
A 37-year-old male patient visited Seoul National University Bundang Hospital for further evaluation of pancytopenia. His complete blood count (CBC) revealed a hemoglobin concentration of 79 g/L, an absolute neutrophil count of 523×106/L, and a platelet count of 69×109/L. Peripheral blood smear showed 26% blasts. Subsequently, BM biopsy was performed, which revealed an increase in myeloblasts positive for CD13, CD33, CD34, CD38 (partial), CD117, HLA-DR, and CD123, as confirmed with flow cytometry. Furthermore, the results revealed hypercellular marrow with dysmegakaryopoietic features such as monolobation, micromegakaryocytes (Fig. 1A), and grade 2 reticulin fibrosis (Fig. 1B). The initial cytogenetic study showed no mitosis. However, the fluorescence in situ hybridization results indicated trisomy 8. Next-generation sequencing (NGS) was performed with NextSeq500 (Illumina, San Diego, CA, USA) using a SureSelect Custom panel including 196 genes commonly mutated in myeloid neoplasms. The NGS analysis resulted in detection of the JAK2, MPL, and ZRSR2 variants (Fig. 2A–C): NM_004972.3(JAK2):c.1849G>T (p.Val617Phe), variant allele frequency (VAF) of 22.2%; NM_005373.2(MPL):c.1543T>A (p.Trp515Arg), VAF of 5.7%; NM_005089.3(ZRSR2):c.1093_1103del (p.Glu365Profs*16), VAF of 60.2%, respectively. The patient had received his first stem cell transplantation in 2018, but relapsed and received a second stem cell transplantation in 2019. Dysmegakaryopoietic features partially remained in the BM biopsy performed after stem cell transplantation (Fig. 3). The patient is currently in a complete remission state.
A 47-year-old male patient with dysarthria was admitted to the neurology department of Seoul National University Bundang Hospital. He had never been diagnosed with hematologic disorders or cancer. Severe left middle cerebral artery stenosis and multifocal territories embolic infarction were confirmed with brain magnetic resonance imaging. The CBC profile of the patient revealed a hemoglobin concentration of 136 g/L, an absolute neutrophil count of 2,320×106/L, and a platelet count of 183×109/L, indicating no cytopenia but presence of more than 20% peripheral blasts. Next, BM biopsy was performed, which revealed an increase in myeloblasts positive for CD13, CD33, CD34, CD117 (partial), HLA-DR, and myeloperoxidase (partial), as confirmed with flow cytometry. Additionally, normocellular marrow with dysmegakaryopoietic features, such as widely separate nuclei and monolobation (Fig. 1C), and grade 2 reticulin fibrosis (Fig. 1D) were also observed. The initial cytogenetic study indicated a normal karyotype. Based on these findings, a diagnosis of AML, not otherwise specified was made. The NGS analysis identified JAK2 V617F with a VAF of 23.7%. An additional mutation, NM_005475.2(SH2B3):c.1009dup (p.Ser337Phefs*3), was found in the SH2B3 gene (Fig. 2D and E). Despite the need for chemotherapy, the patient refused to receive treatment and was discharged.
The valine-to-phenylalanine substitution at codon 617 in the JAK2 protein is a common mutation in BCR-ABL1-negative MPNs. However, this mutation is reported at a low frequency (<5%) in de novo AML [2, 3]. These cases appeared in the literature from 2005 onwards, mostly as case reports or as small case series. Hidalgo-López et al. [4] reported the comparison between de novo AML with JAK2 V617F mutation and age-, sex-, and WHO classification diagnosis-matched JAK2 wild type AML. The JAK2 V617F mutation has been frequently identified in patients with BM dysplasia and myelofibrosis, an abnormal karyotype, and mutations in genes involved in DNA methylation and epigenetic-modifying pathways, and in the absence of gene mutations in activating signaling pathways other than JAK2. In 2018, Aynardi et al. [5] reported the largest-to-date case series, which included 15 patients. They compared de novo AML with the JAK2 V617F mutation and secondary AML transformed from an underlying MPN. Splenomegaly, MPN-like megakaryocytic atypia at the time of AML diagnosis, and complex karyotype were less commonly observed in de novo JAK2 V617F-positive AML compared to those in secondary AML from underlying MPN. Normal karyotype and mutations in the genes encoding DNA methylation mediators were more commonly observed in de novo AML, and the mean JAK2 V617F allele frequency was higher in secondary AML (50%) than in de novo AML (32%). These previous studies did not reveal the specific role of the JAK2 V617F mutation in AML pathogenesis, but proposed that this entity exhibits features distinct from those of secondary AML.
In this case report, we presented two cases of JAK2 V617F-positive AML. The patients were diagnosed with AML without any evidence of antecedent MPN. The presence of dysmegakaryopoiesis and a non-complex karyotype were consistent with the findings of previous studies. Both patients carried the JAK2 V617F mutation and the variant allele frequency was 22.2% and 23.7%. As previous studies reported that mutations in the activating signaling pathways other than JAK2 are rarely observed in de novo JAK2 V617F-positive AML, the additional mutation in the MPL gene was a rare finding. Patient 1 exhibited MPL W515R with a VAF of 5.7%. The coexistence of driver mutations is also a rare event in MPN patients [6, 7]. Most MPL mutations substituted at the amino acid position 515 (W515) reportedly lead to the activation of MPL and result in constitutive activation of JAK2 and STAT protein signaling [8]. It is noteworthy that the allele burden of MPL W515R was significantly lower (5.7%) than that (22.2%) of JAK2 V617F. This could be explained by pre-existing clonal hematopoiesis, followed by the acquisition of a second oncogenic mutation [9]. Considering that Patient 2 visited the hospital with a history of cerebral infarction and no previous diagnosis was established, he could potentially have underlying MPN. However, in previous reports, JAK2 V617F allele frequency tended to be lower in de novo JAK2 V617F-positive AML than in secondary AML [5]. The JAK2 V617F allele frequency of Patients 1 and 2 was 22.2 and 23.7%, respectively, which could be considered low.
JAK2 V617F-positive AML is a distinct biological entity from secondary AML transformed from underlying MPNs. Presence of the JAK2 V617F mutation in AML does not imply a blast phase of an occult MPN. The cases described here support the presence of this unique entity. In patients newly diagnosed with JAK2 V617F-positive AML without a history of previously diagnosed hematologic disease, dysmegakaryopoiesis, myelofibrosis, and non-complex karyotype are common. Splenomegaly and the mean VAF of the JAK2 mutation are also important in discriminating between de novo JAK2 V617F-positive AML and secondary AML progressed from antecedent MPN.
Acknowledgments
This study was supported by the research fund from the Seoul National University Bundang Hospital (No. 16-2017-006).
REFERENCES
1. Swerdlow SH, Campo E, editors. 2017. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th ed. Lyon, France: International Agency for Research on Cancer. p. 39–53.
2. Steensma DP, McClure RF, Karp JE, Tefferi A, Lasho TL, Powell HL, et al. 2006; JAK2 V617F is a rare finding in de novo acute myeloid leukemia, but STAT3 activation is common and remains unexplained. Leukemia. 20:971–8. DOI: 10.1038/sj.leu.2404206. PMID: 16598306.
3. Lee JW, Kim YG, Soung YH, Han KJ, Kim SY, Rhim HS, et al. 2006; The JAK2 V617F mutation in de novo acute myelogenous leukemias. Oncogene. 25:1434–6. DOI: 10.1038/sj.onc.1209163. PMID: 16247455.
4. Hidalgo- López JE, Kanagal-Shamanna R, L. Medeiros LJ, Estrov Z, Yin CC, Verstovsek S, et al. 2017; Morphologic and molecular characteristics of de novo AML with JAK2 V617F mutation. J Natl Compr Canc Netw. 15:790–6. DOI: 10.6004/jnccn.2017.0106. PMID: 28596259.
5. Aynardi J, Manur R, Hess PR, Chekol S, Morrissette JJD, Babushok D, et al. 2018; JAK2 V617F-positive acute myeloid leukemia (AML): a comparison between de novo AML and secondary AML transformed from an underlying myeloproliferative neoplasm. A study from the Bone Marrow Pathology Group. Br J Haematol. 182:78–85. DOI: 10.1111/bjh.15276. PMID: 29767839.
6. De Roeck L, Michaux L, Debackere K, Lierman E, Vandenberghe P, Devos T. 2018; Coexisting driver mutations in MPN: clinical and molecular characteristics of a series of 11 patients. Hematology. 23:785–92. DOI: 10.1080/10245332.2018.1498182. PMID: 29993347.

7. Jang MA, Seo MY, Choi KJ, Hong DS. 2020; A rare case of essential thrombocythemia with coexisting JAK2 and MPL driver mutations. J Korean Med Sci. 35:e168. DOI: 10.3346/jkms.2020.35.e168. PMID: 32537949. PMCID: PMC7295601.

8. Defour JP, Chachoua I, Pecquet C, Constantinescu SN. 2016; Oncogenic activation of MPL/thrombopoietin receptor by 17 mutations at W515: implications for myeloproliferative neoplasms. Leukemia. 30:1214–6. DOI: 10.1038/leu.2015.271. PMID: 26437785.

9. Mansier O, Luque Paz D, Ianotto JC, Le Bris Y, Chauveau A, Boyer F, et al. 2018; Clinical and biological characterization of MPN patients harboring two driver mutations, a French intergroup of myeloproliferative neoplasms (FIM) study. Am J Hematol. 93:E84–6. DOI: 10.1002/ajh.25014. PMID: 29266414.

Fig. 1
Morphological features of the bone marrow from patients with AML carrying the JAK2 V617F mutation. (A) Patient 1: Micromegakaryocytes (hematoxylin-eosin, 400×). (B) Patient 1: diffuse reticulin fibrosis (MF-2) (reticulin, 400×). (C) Patient 2: Hypolobated, dysplastic megakaryocytes (hematoxylin-eosin, 400×). (D) Patient 2: diffuse reticulin fibrosis (MF-2) (reticulin, 200×).

Fig. 2
IGV browser visualization of the NGS results. (A) Patient 1: NM_004972.3(JAK2):c.1849G>T (p.Val617Phe) in the JAK2 gene. (B) Patient 1: NM_005373.2(MPL):c.1543T>A (p.Trp515Arg) in the MPL gene. (C) Patient 1: NM_005089.3(ZRSR2):c.1093_1103del (p.Glu365Profs*16) in the ZRSR2 gene. (D) Patient 2: NM_004972.3(JAK2):c.1849G>T (p.Val617Phe) in the JAK2 gene. (E) Patient 2: NM_005475.2(SH2B3):c.1009dup (p.Ser337Phefs*3) in the SH2B3 gene.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download