Anti-Müllerian hormone (AMH) is produced by the ovarian granulosa cells after birth until menopause. Its levels serve as an index that reflects ovarian reserve. AMH levels, along with antral follicle count, have become a useful tool for the assessment of reproductive potential [
1]. Female fertility is known to be affected by various factors other than age, including smoking (including passive smoking), excessive alcohol intake, stress, poor diet, athletic training, being overweight or underweight, drug use, and particular occupations that involve exposure to hazards [
1,
2]. Recent lifestyle changes in Korea, including the gradual increase in smoking and binge drinking, and the economic activity of women, may contribute to a decrease in female fertility. Additionally, the methodology for the AMH test has also changed over time, leading to differences in the measured AMH levels [
3,
4]. Therefore, it is necessary to examine whether the reference values used are appropriate and updated. The purpose of this study was to examine the changes in the AMH levels in Korean women with normal menstruation to understand the trends in female fertility over time. Additionally, we reviewed whether the current age-specific reference values for AMH levels are clinically suitable, using recent databases that include large-scale populations.
We collected data of the AMH levels and distribution variables (corresponding percentile interval and median age for each serum AMH value, calculated using the algorithm programmed by the Hamchoon Institute of Fertility & Genetics) from the records collected by Seoul Clinical Laboratories between 2015 and 2021. From January 2015 to March 2018, the serum AMH levels were measured using the AMH Gen II enzyme–linked immunosorbent assay (ELISA) (A79765; Beckman Coulter, Immunotech, Webster, TX, USA). After April 2018, the Access AMH assay (Beckman Coulter, Brea, CA, USA) was used for measuring the AMH levels using a Beckman Coulter UniCel DxI 800 system (Beckman Coulter, Fullerton, CA, USA). Both immunoassays used an identical pair of antibodies and presented results in ng/mL; however, their characteristics differed based on the manufacturer’s instructions. The analysis algorithm determined the distribution variables and divided the data into the 10th, 30th, 50th, 70th, and 90th percentile intervals of serum AMH concentration, for women aged 25–48 years. On this basis, age-specific reference values were assigned for each of the two assays.
Age, sex, race, menstrual habit, and menstrual cycle data of study population were available from the laboratory center’s data registry. Records of the AMH levels of Korean women, aged 20–49 years, who had regular menstrual cycles with an interval of 21 to 35 days and a duration of 3 to 7 days, were included in this study. We excluded non-Korean women, patients with missing elements of data, and duplicate data, so as to include only one record for each subject. This study was granted exemption from review by the institutional review board of Seoul Clinical Laboratories (IRB-21-001).
All analyses were performed using the online GraphPad Prism software, version 5.0 (GraphPad Software, Inc., San Diego, CA, USA) and R version 4.0.2 software (R Foundation for Statistical Computing, Vienna, Austria). Continuous data is presented as the medians with interquartile range. Data were compared by analysis of variance (ANOVA), Tukey’s honestly significant difference test, and Kruskal-Wallis Rank Sum test. P values of <0.05 were considered statistically significant.
A total of 33,529 AMH level measurements were included in this study (N=19,032 for Gen II assay; N=14,497 for Access assay).
Table 1 summarizes the baseline characteristics of the study population for each AMH assay. There was a significant difference between the ages and AMH levels (
P<0.001) recorded using each assay. The two-way ANOVA test revealed significant differences between the year that the test was conducted and the AMH level, only in the case of the Gen II assay (Gen II assay:
P<0.001; Access assay:
P=0.880). Specifically, the AMH levels in patients of all ages (20–49 years) and those in their early 40s (40–44 years) differed significantly between 2015 and 2016 (
P=0.0060 and
P<0.001, respectively) and between 2015 and 2017 (
P<0.001 and
P=0.034, respectively) (
Fig. 1A, B).
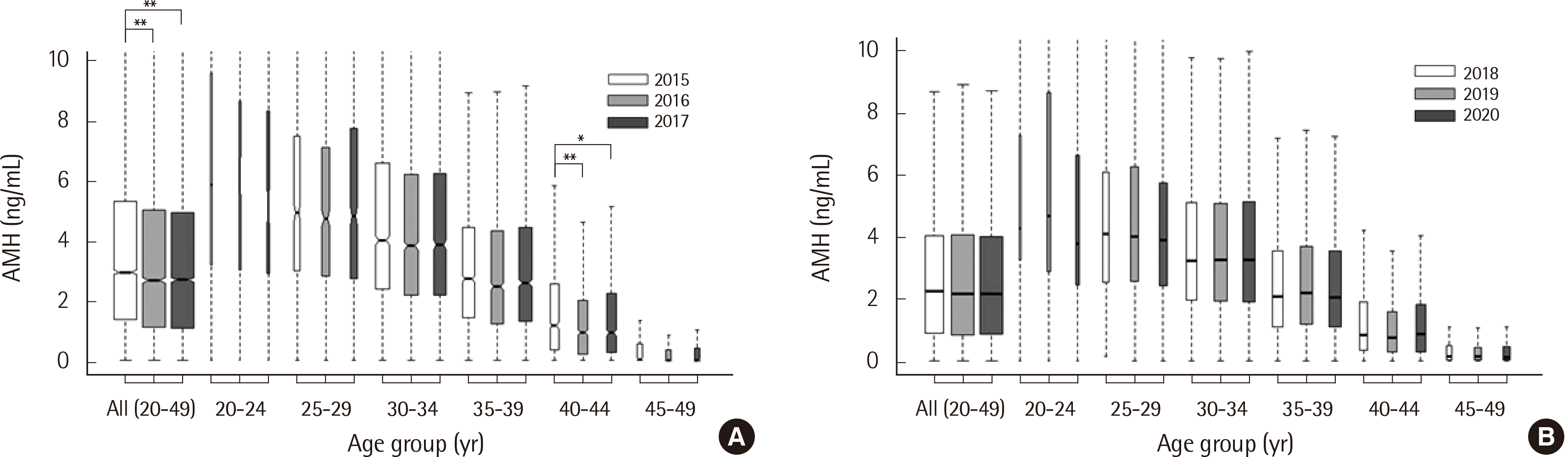 | Fig. 1Levels of anti-Müllerian hormone (AMH) in Korean women with normal menstruation by (A) Gen II assay (N=19,032) and (B) Access assay (N=13,078). *P<0.05, **P<0.001. 
|
Table 1
The baseline characteristics of the study population for each anti-Müllerian hormone assay
|
Year |
Parameter |
Age group (yr) |
|
|
All (20–49) |
20–24 |
25–29 |
30–34 |
35–39 |
40–44 |
45–49 |
|
Gen II assay |
|
|
|
|
|
|
|
|
|
|
2015 |
N |
|
6,375 |
59 |
558 |
2,426 |
2,032 |
937 |
363 |
|
Age (yr) |
Median |
35 |
23 |
28 |
32 |
36 |
41 |
46 |
|
|
IQR |
32–38 |
22–24 |
27–29 |
31–33 |
35–38 |
40–43 |
45–48 |
|
AMH (ng/mL) |
Median |
2.99 |
5.89 |
4.97 |
4.05 |
2.77 |
1.23 |
0.12 |
|
|
IQR |
1.42–5.33 |
3.25–9.58 |
3.04–7.50 |
2.45–6.62 |
1.49–4.47 |
0.42–2.60 |
0.08–0.61 |
|
2016 |
N |
|
6,493 |
54 |
615 |
2,430 |
2,026 |
978 |
390 |
|
Age (yr) |
Median |
35 |
23 |
28 |
32 |
37 |
42 |
46 |
|
|
IQR |
32–39 |
22–24 |
27–29 |
31–33 |
35–38 |
40–43 |
45–48 |
|
AMH (ng/mL) |
Median |
2.68 |
6.19 |
4.77 |
3.87 |
2.52 |
1.00 |
0.08 |
|
|
IQR |
1.16–4.98 |
3.10–8.64 |
2.88–7.14 |
2.23–6.24 |
1.29–4.37 |
0.29–2.06 |
0.08–0.41 |
|
2017 |
N |
|
6,164 |
68 |
586 |
2,045 |
2,055 |
991 |
419 |
|
Age (yr) |
Median |
35 |
23 |
28 |
32 |
37 |
42 |
46 |
|
|
IQR |
32–39 |
22–24 |
27–29 |
31–33 |
35–38 |
40–43 |
45–48 |
|
AMH (ng/mL) |
Median |
2.74 |
5.17 |
4.86 |
3.91 |
2.63 |
0.99 |
0.09 |
|
|
IQR |
1.15–4.98 |
2.98–8.29 |
2.79–7.75 |
2.24–6.26 |
1.36–4.49 |
0.33–2.30 |
0.08–0.49 |
|
Access assay |
|
|
|
|
|
|
|
|
|
|
2018 |
N |
|
3,160 |
59 |
340 |
981 |
983 |
527 |
270 |
|
Age (yr) |
Median |
36 |
23 |
28 |
32 |
37 |
42 |
46 |
|
|
IQR |
32–40 |
22–24 |
26–29 |
31–33 |
36–38 |
41–43 |
45–48 |
|
AMH (ng/mL) |
Median |
2.25 |
4.29 |
4.10 |
3.24 |
2.09 |
0.86 |
0.17 |
|
|
IQR |
0.91–4.04 |
3.26–7.27 |
2.56–6.08 |
1.98–5.12 |
1.12–3.57 |
0.36–1.91 |
0.05–0.50 |
|
2019 |
N |
|
5,000 |
90 |
550 |
1,548 |
1,498 |
837 |
477 |
|
Age (yr) |
Median |
36 |
23 |
28 |
32 |
37 |
42 |
46 |
|
|
IQR |
32–40 |
22–24 |
27–29 |
31–33 |
36–38 |
41–43 |
45–48 |
|
AMH (ng/mL) |
Median |
1.42 |
4.69 |
4.01 |
3.26 |
2.21 |
0.76 |
0.17 |
|
|
IQR |
0.48–3.02 |
2.92–8.65 |
2.59–6.26 |
1.96–5.08 |
1.21–3.71 |
0.31–1.61 |
0.04–0.46 |
|
2020 |
N |
|
4,918 |
126 |
596 |
1,478 |
1,419 |
856 |
443 |
|
Age (yr) |
Median |
35 |
23 |
28 |
32 |
37 |
42 |
46 |
|
|
IQR |
31–40 |
21–24 |
27–29 |
31–33 |
36–38 |
40–43 |
45–48 |
|
AMH (ng/mL) |
Median |
1.44 |
3.79 |
3.91 |
3.28 |
2.07 |
0.88 |
0.14 |
|
|
IQR |
0.49–2.78 |
2.48–6.61 |
2.45–5.75 |
1.92–5.15 |
1.10–3.57 |
0.31–1.82 |
0.04–0.49 |

We summarized the medians of the analyzed variables for the AMH levels using the algorithm programmed by Hamchoon Institute of Fertility & Genetics for AMH levels, as shown in
Table 2. The results of both assays showed that the percentile intervals of the groups at both age extremes (late 20s and late 40s) were lower than those of the middle age groups (30s and early 40s) (30–50th percentiles versus 50–70th percentiles). Results of the Gen II assay revealed that the percentile intervals of subjects in their late 40s (45–49 years) showed a negative deviation from the median value (50th percentile). The median age of subjects in their late 20s (25–29 years) showed a relatively large deviation from the actual age.
Table 2
Analyzed variables for anti-Müllerian hormone levels, using the algorithm programmed by the Hamchoon Institute of Fertility & Genetics
|
Age① (yr) |
Gen II assay |
Access assay |
|
|
|
N |
Median percentile interval*
|
Median age② with the same level |
Difference in age (①–②) |
N |
Median percentile interval*
|
Median age③ with the same level |
Difference in age (①–③) |
|
25 |
122 |
30–50 |
30 |
-5 |
134 |
30–50 |
25 |
0 |
|
26 |
199 |
30–50 |
31 |
-5 |
192 |
50–70 |
25 |
1 |
|
27 |
306 |
30–50 |
30 |
-3 |
289 |
30–50 |
28 |
-1 |
|
28 |
438 |
30–50 |
31 |
-3 |
391 |
30–50 |
29 |
-1 |
|
29 |
694 |
30–50 |
31 |
-2 |
480 |
30–50 |
30 |
-1 |
|
30 |
977 |
30–50 |
31 |
-1 |
674 |
30–50 |
31 |
-1 |
|
31 |
1,210 |
30–50 |
32 |
-1 |
760 |
30–50 |
31 |
0 |
|
32 |
1,459 |
50–70 |
32 |
0 |
831 |
30–50 |
33 |
-1 |
|
33 |
1,552 |
50–70 |
33 |
0 |
860 |
30–50 |
34 |
-1 |
|
34 |
1,703 |
50–70 |
34 |
0 |
882 |
50–70 |
34 |
0 |
|
35 |
1,601 |
50–70 |
35 |
0 |
827 |
50–70 |
35 |
0 |
|
36 |
1,425 |
50–70 |
35 |
1 |
851 |
50–70 |
36 |
0 |
|
37 |
1,176 |
50–70 |
36 |
1 |
817 |
50–70 |
36 |
1 |
|
38 |
1,072 |
50–70 |
37 |
1 |
771 |
50–70 |
37 |
1 |
|
39 |
839 |
50–70 |
38 |
1 |
634 |
50–70 |
38 |
1 |
|
40 |
785 |
50–70 |
38 |
2 |
557 |
50–70 |
40 |
0 |
|
41 |
604 |
50–70 |
40 |
1 |
459 |
50–70 |
40 |
1 |
|
42 |
563 |
50–70 |
42 |
0 |
481 |
50–70 |
42 |
0 |
|
43 |
535 |
50–70 |
43 |
0 |
366 |
30–50 |
43 |
0 |
|
44 |
419 |
50–70 |
43 |
1 |
357 |
30–50 |
45 |
-1 |
|
45 |
355 |
10–30 |
47 |
-2 |
310 |
30–50 |
46 |
-1 |
|
46 |
277 |
0–10 |
48 |
-2 |
287 |
30–50 |
47 |
-1 |
|
47 |
230 |
0–10 |
48 |
-1 |
225 |
30–50 |
48 |
-1 |
|
48 |
189 |
0–10 |
48 |
0 |
209 |
30–50 |
48 |
0 |

Age-specific percentile values and centile charts of the AMH levels determined using the Access assay in Korean women, aged 25–49 years, with normal menstruation were calculated; at least 120 subjects of each age category were included (
Table 3). Additionally, we found separate reference values for the age and age ranges using the 2.5th to 97.5th percentiles (central 95 percentiles) of the AMH levels in Korean women with normal menstruation. Finally, we evaluated the new age-specific reference values and percentile values based on the Clinical and Laboratory Standards Institute EP28–A3c [
3], and the clinical suitability of them by comparing the medians of the low and high percentiles of the new and current AMH percentile values determined by the Access assay in Korean women with normal menstruation (January to April 2021, N=1,419). The new age-specific reference values were verified for most of age, and the new age-specific percentiles for subjects interpreted as healthy and reproductive were close to the median value (50th percentile) (
Table 3,
Fig. 2).
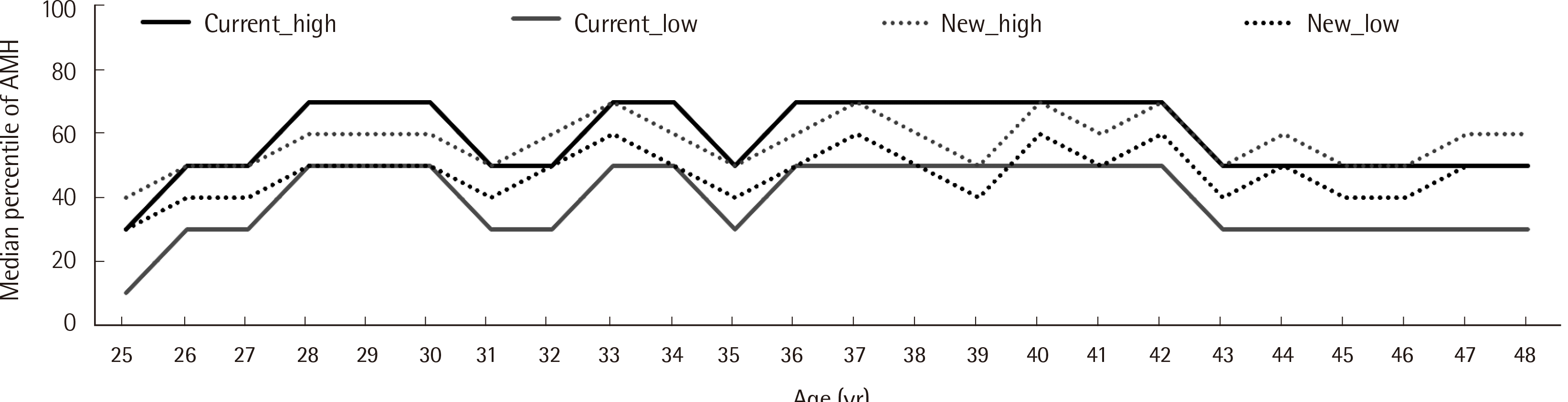 | Fig. 2Medians of low and high percentiles applied current and new AMH percentiles for Access assay in Korean women with normal menstruation (Same population with ‘RV verification’ in table 3). 
|
Table 3
Age-specific anti-Müllerian hormone levels in Korean women with normal menstruation, assessed using the Access assay
|
Age (yr)*
|
Serum AMH percentiles (ng/mL) |
Reference values: 2.5–97.5 percentiles |
Reference value verification†
|
|
|
|
10th |
20th |
30th |
40th |
50th |
60th |
70th |
80th |
90th |
Result (N) |
Outside N (%) |
|
|
25 |
1.65 |
2.46 |
3.05 |
3.63 |
4.12 |
4.88 |
5.94 |
7.49 |
9.30 |
0.73–16.92 |
Not completed (19) |
1 (5.26) |
|
26 |
1.73 |
2.49 |
2.95 |
3.65 |
4.38 |
5.03 |
6.27 |
7.26 |
9.79 |
0.86–14.06 |
Not completed (22) |
3 (13.64) |
|
27 |
1.63 |
2.24 |
2.83 |
3.47 |
4.01 |
4.57 |
5.44 |
6.50 |
8.95 |
0.68–12.55 |
Verified (39) |
1 (2.56) |
|
28 |
1.74 |
2.30 |
2.78 |
3.36 |
4.05 |
4.60 |
5.39 |
6.41 |
8.10 |
0.81–12.37 |
Verified (64) |
5 (7.81) |
|
29 |
1.39 |
2.01 |
2.57 |
3.24 |
3.79 |
4.50 |
5.26 |
6.21 |
7.92 |
0.79–11.96 |
Verified (62) |
3 (4.84) |
|
30 |
1.46 |
2.00 |
2.53 |
3.14 |
3.70 |
4.38 |
5.26 |
6.44 |
8.53 |
0.60–11.47 |
Verified (86) |
3 (3.49) |
|
31 |
1.21 |
1.87 |
2.43 |
3.01 |
3.65 |
4.23 |
5.03 |
5.99 |
7.80 |
0.47–11.40 |
Verified (88) |
5 (5.68) |
|
32 |
1.14 |
1.77 |
2.26 |
2.74 |
3.25 |
3.85 |
4.65 |
5.77 |
7.36 |
0.46–10.75 |
Verified (104) |
7 (6.73) |
|
33 |
1.09 |
1.57 |
2.06 |
2.53 |
3.06 |
3.56 |
4.28 |
5.26 |
6.73 |
0.45–10.01 |
Verified (90) |
2 (2.22) |
|
34 |
0.86 |
1.38 |
1.85 |
2.31 |
2.90 |
3.46 |
4.09 |
4.98 |
6.39 |
0.30–9.47 |
Verified (89) |
2 (2.25) |
|
35 |
0.92 |
1.35 |
1.74 |
2.22 |
2.75 |
3.20 |
3.81 |
4.69 |
6.09 |
0.26–8.38 |
Verified (72) |
6 (8.33) |
|
36 |
0.76 |
1.17 |
1.51 |
1.89 |
2.29 |
2.86 |
3.50 |
4.24 |
5.78 |
0.25–9.12 |
Verified (68) |
2 (2.94) |
|
37 |
0.51 |
0.87 |
1.28 |
1.68 |
2.17 |
2.75 |
3.33 |
3.95 |
5.15 |
0.13–7.18 |
Verified (63) |
4 (6.35) |
|
38 |
0.44 |
0.77 |
1.16 |
1.53 |
1.82 |
2.32 |
3.00 |
3.81 |
5.16 |
0.11–8.47 |
Verified (84) |
5 (5.95) |
|
39 |
0.32 |
0.66 |
0.93 |
1.22 |
1.59 |
1.94 |
2.45 |
3.15 |
4.04 |
0.07–6.40 |
Verified (86) |
5 (5.81) |
|
40 |
0.21 |
0.51 |
0.77 |
1.02 |
1.28 |
1.54 |
1.95 |
2.56 |
3.56 |
≤ 5.40 |
Verified (66) |
0 (0.00) |
|
41 |
0.16 |
0.38 |
0.56 |
0.81 |
1.09 |
1.54 |
2.07 |
2.57 |
3.62 |
≤ 5.60 |
Verified (47) |
0 (0.00) |
|
42 |
0.09 |
0.22 |
0.40 |
0.57 |
0.78 |
1.07 |
1.43 |
1.92 |
2.82 |
≤ 4.95 |
Verified (43) |
2 (4.65) |
|
43 |
0.05 |
0.14 |
0.30 |
0.44 |
0.57 |
0.79 |
1.11 |
1.54 |
2.28 |
≤ 4.15 |
Verified (62) |
2 (3.23) |
|
44 |
0.02 |
0.06 |
0.14 |
0.24 |
0.38 |
0.50 |
0.68 |
1.13 |
1.73 |
≤ 3.02 |
Not completed (36) |
4 (11.11) |
|
45 |
0.02 |
0.06 |
0.11 |
0.19 |
0.31 |
0.47 |
0.63 |
0.92 |
1.43 |
≤ 2.47 |
Verified (31) |
1 (3.23) |
|
46 |
0.02 |
0.04 |
0.07 |
0.13 |
0.21 |
0.30 |
0.44 |
0.62 |
1.11 |
≤ 1.77 |
Verified (33) |
0 (0.00) |
|
47 |
0.02 |
0.04 |
0.05 |
0.09 |
0.13 |
0.19 |
0.29 |
0.53 |
0.78 |
≤ 1.42 |
Verified (38) |
2 (5.26) |
|
48 |
0.02 |
0.02 |
0.04 |
0.06 |
0.12 |
0.17 |
0.21 |
0.35 |
0.55 |
≤ 1.16 |
Verified (25) |
1 (4.00) |
|
49 |
0.02 |
0.02 |
0.03 |
0.04 |
0.06 |
0.10 |
0.17 |
0.25 |
0.44 |
≤ 1.00 |
Not completed (17) |
1 (5.88) |
|
|
Age range (yr) |
N |
Reference values: 2.5–97.5 percentiles |
Reference value verification†
|
|
|
Result (N) |
Outside N (%) |
|
25–29 |
1,486 |
0.75–13.38 |
Verified (206) |
11 (5.34) |
|
30–34 |
4,007 |
0.41–10.64 |
Verified (457) |
18 (3.94) |
|
35–39 |
3,900 |
0.12–8.18 |
Verified (373) |
20 (5.36) |
|
40–44 |
2,220 |
≤ 4.91 |
Verified (254) |
7 (2.76) |
|
45–49 |
1,190 |
≤ 1.93 |
Verified (127) |
4 (3.15) |

The AMH levels measured by the Gen II assay and Access assay differed significantly (
P<0.001). Similar to the results of previous studies [
4,
5], the median AMH levels measured by the Access assay were lower than those measured using the Gen II assay for all age groups, except for the late 40s group (45–49 years) (
Table 1). The Gen II ELISA assay (original assay) produced considerably lower values than expected, as a result of its complement interference; therefore, Beckman Coulter revised it by adding a predilution step (modified Gen II assay) [
6]. In this study, the modified Gen II assay was used, and the sample was prepared (30 µL sample+150 µL AMH Gen II assay buffer) prior to conducting the assay. Unlike the modified Gen II assay, which uses solid-phase ELISA, the automated Access assay detects liquid-phase antigen-antibody reactions in a 1:9 prediluted sample and is based on the principle of chemiluminescence. These factors possibly contribute to the differences in the measured values between the two methods.
In analyzing the AMH data for each assay, we found no significant changes in the AMH levels over time, except for a specific age group in the Gen II assay group. Therefore, the decrease in AMH levels during the last 6 years may be attributed to the changes in the AMH assay. No specific trends of AMH levels with time were observed.
The analysis algorithm programmed by the Hamchoon Institute of Fertility & Genetics has been used in the interpretation of serum AMH results since 2015. It is used to predict AMH values based on age, using an AMH-age regression model [
7,
8]. The algorithm showed that the median values of the age-specific percentile intervals at both age extremes were relatively low in both assays, especially in the Gen II assay. In other words, the serum AMH levels in Korean women with normal menstruation at both age extremes were low, based on current age-specific reference values. One possible explanation for this may be that the regression model included data of subjects who visited infertility centers, a relatively small number of whom were in their late 20s or late 40s (age extremes in this study).
We proposed age-specific reference values for the Access AMH assay based on the data collected from Korean women with normal menstruation. Our study population differed from that of previous studies [
8–
10], as it included a large sample size (N=12,803), was not limited to patients visiting infertility or women’s health clinics, and included sufficient subjects from both age extremes. We also verified the new reference values for each age and age range with additional clinical data. Therefore, our findings can help in the interpretation of serum AMH levels of Korean women with normal menstruation. This study did have certain limitations. As the inclusion criteria for this study were based on menstrual history, the subjects included in the study may not have been heal-thy otherwise. In addition, the AMH levels were not compared with other indicators of ovarian reserve.
We determined that the AMH levels of Korean women did not show a time trend, except in the context of the test method used. In the case of the Gen II assay, the current age-specific AMH reference values, based on an AMH-age regression model, may not be clinically suitable for patients at the two age extremes. Moreover, the manufacturer’s instructions only provide reference values for age ranges for the Access AMH assay conducted using the Access 2 system. Therefore, we established new age-specific AMH reference values (2.5–97.5th percentiles and percentiles of 10 intervals) for the general population, measured using the Access assay on the DXI800 system.
Go to :







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download