1. Mujahid A, Dickert FL. 2015; Blood group typing: from classical strategies to the application of synthetic antibodies generated by molecular imprinting. Sensors (Basel). 16:51. DOI:
10.3390/s16010051. PMID:
26729127. PMCID:
PMC4732084.

3. Kim S, Song S, Kim HO. 2018; Cryopreservation of genotyped ABO subgroup RBCs for quality assurance of ABO grouping reagents. Ann Clin Lab Sci. 48:216–22. PMID:
29678850.
4. Lim HH, Kim KH, An GD, Jeong IH, Son YK. 2018; Acute hemolytic transfusion reaction due to ABO-incompatible blood transfusion: a fatal case report and review of the literature. Korean J Blood Transfus. 29:73–8. DOI:
10.17945/kjbt.2018.29.1.73.

7. India National Institute of Biologicals. 2012. Guidance Manual-Quality control of ABO and Rh blood grouping reagents. Document ID No. NIB/BRL /GM/01. NOIDA. India Ministry of Health and Family Welfare;NOIDA:
11. Lee JY, Oh DJ, park YM. 2010; The frequency and distribution of the ABO subgroups in Korean blood donors. Korean J Blood Transfus. 21:223–9.
12. Yang HS, Chun S, Lee SA, Kwon JR, Choi YS, Kim JN, et al. 2017; Transfusion strategy of RhD-negative/variant patients in the Korean population. Lab Med Online. 7:89–93. DOI:
10.3343/lmo.2017.7.3.89.

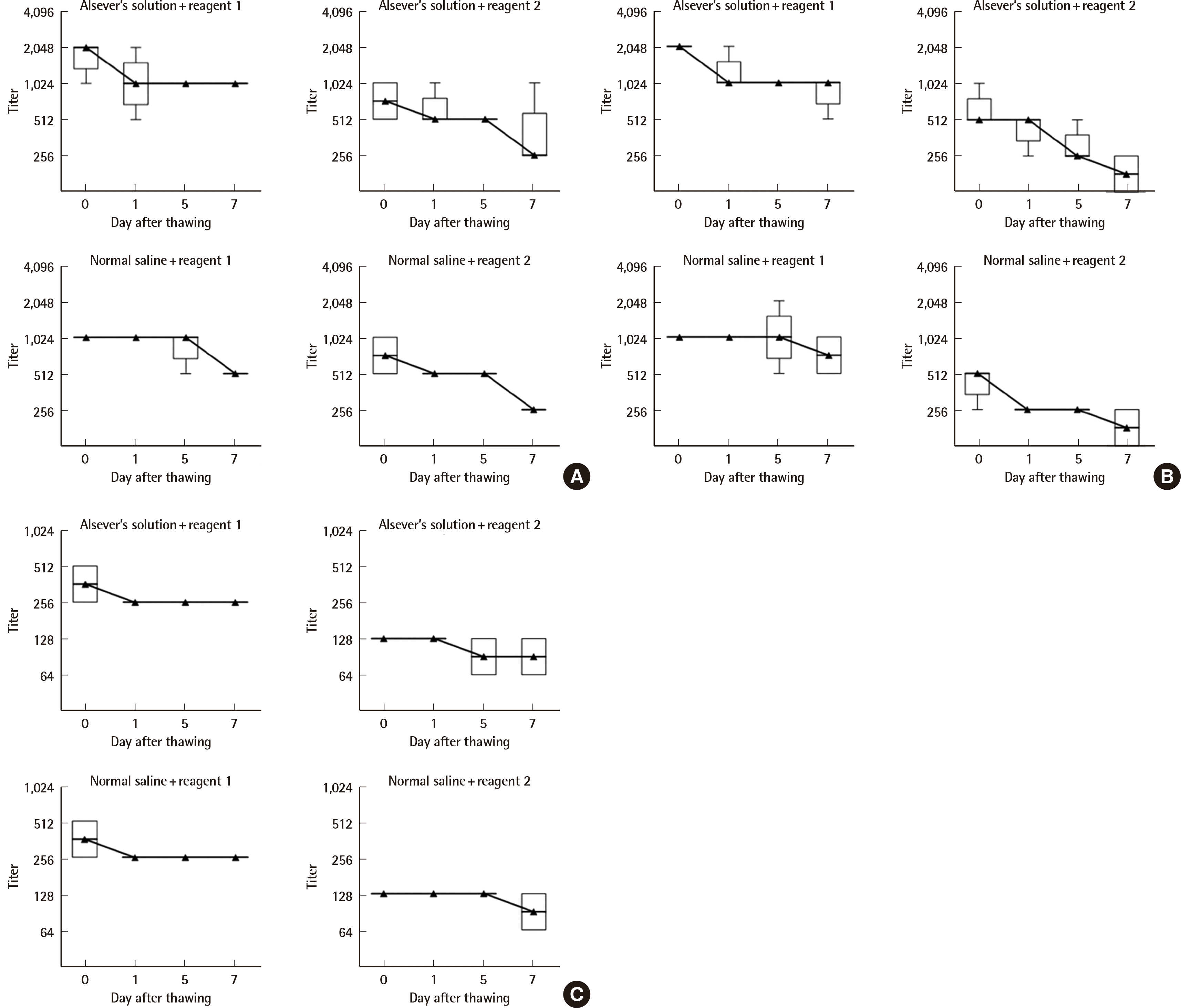
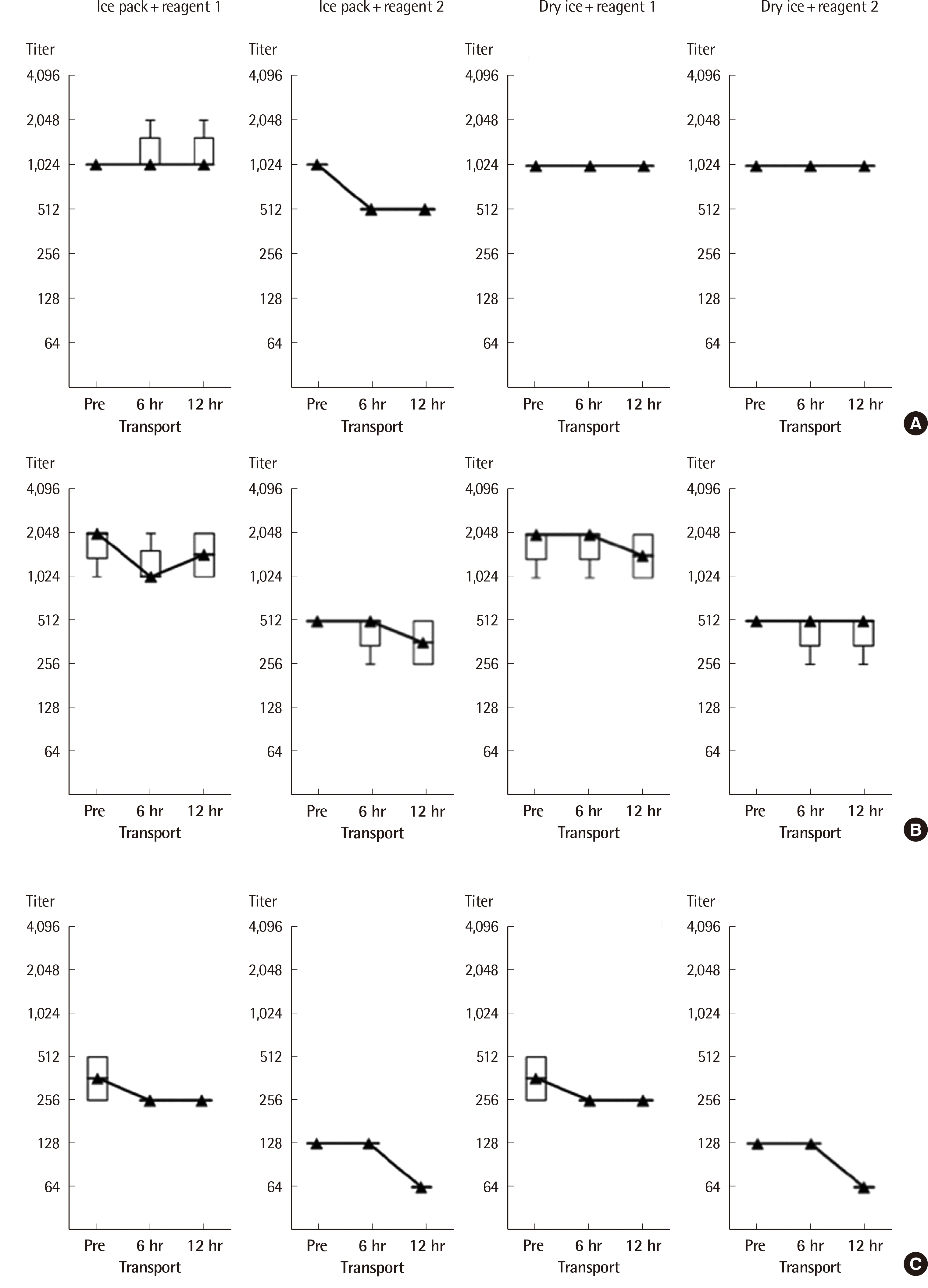
13. Song S, Choi J, Kim S, Kim HO, Min H, Kim J, et al. 2009; Development of cryopreserved red blood cell panels for verifying ABO and D blood grouping reagents. Korean J Blood Transfus. 20:46–54.
15. Högman CF, Hornblower ML, Flodin M, Gillberg G, Meryman HT, Säfwenberg J. 1986; A simple method for high-quality frozen red cells in blood group serology. Transfusion. 26:434–6. DOI:
10.1046/j.1537-2995.1986.26587020120.x. PMID:
3765037.

16. Lelkens CC, Noorman F, Koning JG, Truijens-de Lange R, Stekkinger PS, Bakker JC, et al. 2003; Stability after thawing of RBCs frozen with the high- and low-glycerol method. Transfusion. 43:157–64. DOI:
10.1046/j.1537-2995.2003.00293.x. PMID:
12559010.

18. Gorakshakar A, Gogri H, Ghosh K. 2017; Evolution of technology for molecular genotyping in blood group systems. Indian J Med Res. 146:305–15. DOI:
10.4103/ijmr.IJMR_914_16. PMID:
29355136. PMCID:
PMC5793464.
21. Lee E, Sivalingam J, Lim ZR, Chia G, Shi LG, Roberts M, et al. 2018; Review: in vitro generation of red blood cells for transfusion medicine: progress, prospects and challenges. Biotechnol Adv. 36:2118–28. DOI:
10.1016/j.biotechadv.2018.09.006. PMID:
30273713.

23. Rudmann SV. 2005. Blood component preservation and storage. Textbook of blood banking and transfusion medicine. 2nd ed. Philladelpia, PA: Elsevier Health Sciences. p. 274–5.
24. Valeri CR, Pivacek LE, Gray AD, Cassidy GP, Leavy ME, Dennis RC, et al. 1989; The safety and therapeutic effectiveness of human red cells stored at -80 degrees C for as long as 21 years. Transfusion. 29:429–37. DOI:
10.1046/j.1537-2995.1989.29589284145.x. PMID:
2734823.
25. Valeri CR, Ragno G, Pivacek LE, Cassidy GP, ey R Sr, Hansson-Wicher M, et al. 2000; An experiment with glycerol-frozen red blood cells stored at -80 degrees C for up to 37 years. Vox Sang. 79:168–74. DOI:
10.1046/j.1423-0410.2000.7930168.x. PMID:
11111236.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download