TO THE EDITOR: Secondary/therapy-related neoplasms, such as acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS), occur infrequently in adult patients with acute lymphoblastic leukemia (ALL) [1]. Most frequently, therapy-related/secondary myeloid neoplasms are associated with breast cancer and lymphoproliferative diseases [2]. These patients frequently have complex karyotype including many structural abnormalities indicating a poor prognosis [3]. Here, we report a case of secondary AML in an adult patient with B-ALL on maintenance chemotherapy with an unusual complex karyotype.
A 60-year-old female presented in December 2015 with fever, generalized weakness, and dizziness in the last 2 months. On ultrasonography, she had mild splenomegaly. Complete blood count showed hemoglobin of 77 g/L, total leukocyte counts of 2.84×109/L and platelets of 70×109/L with 2% blasts in peripheral blood smear. Bone marrow aspirate smears were hemodiluted, showing fairly cellular imprint smears with 80% blasts (Fig. 1A). Bone marrow biopsy (Fig. 1B) showed sheets of blasts which are positive for CD10 (Dako, 56C6), PAX5 (Biogenix, ZP007) and negative for MPO and CD33 (Bio SB, RBT-CD33). On flow cytometric immunophenotyping (Fig. 1C), these blasts were positive for CD19, CD10, CD34, HLA-DR, CD22, CD71 (dim), and CD20 (partial), while they were negative for cCD3, cMPO, CD7, CD13, CD33, CD14, CD56, CD4, CD8, CD5, and CD3; these results were consistent with the diagnosis of precursor B-cell acute lymphoblastic leukemia (ALL). Karyotype at this time showed 46,XX. Real-time polymerase chain reaction for t(1;19)(q23 ;p13.3) or TCF-3-PBX1(E2A-PBX1), t(11;19)(q23;p13.3) or MLL-ENL, t(12;21) (p13;q22) or ETV6-RUNX1(TEL-AML1), t(4;11)(q21;q23) or MLL-AF4, t(9;11)(p21-22;q23) or (MLL-AF9), and BCR-ABL1 were negative. She was started on chemotherapy according to the UK ALL protocol. The chemotherapeutic drugs included in the UK ALL treatment regimen were vincristine, daunorubicin, L-asparaginase, prednisolone, and intrathecal methotrexate. Bone marrow after induction therapy was in morphological remission and minimal residual disease by flow cytometry was negative (<0.01%). In February 2017, bone marrow after consolidation therapy was also in morphological remission and minimal residual disease by flow cytometry was negative, and she was started on maintenance chemotherapy. However, in February 2019, she presented with persistent cough. Complete blood count showed hemoglobin of 85 g/L, total leukocyte count of 1.19×109/L, and platelet count of 68×109/L with peripheral blood smear showing 15% blasts. Bone marrow aspirate showed 50% blasts (Fig. 2A) which are larger in size and have abundant granular cytoplasm. On bone marrow biopsy (Fig. 2B), these blasts were positive for CD34 (Dako, QBEnd10), CD33 (Bio SB, RBT-CD33), and CD117 (Biogenix, YR145), and negative for CD10 (Dako, 56C6), CD79a (Dako, JCB117), and PAX5 (Biogenix, ZP007). On flow cytometry (Fig. 1D), these blasts were positive for CD45, CD34, CD13, CD33, CD117 (partial), HLA-DR, CD38, and CD19 and negative for cCD3, cCD79a, CD7, CD22, CD10, CD14, CD64, CD123, CD11b, CD56, and CD58, consistent with the diagnosis of AML. Karyotype, which in December 2015 showed 46,XX, now showed a highly complex karyotype (Fig. 3A). Real-time quantitative PCR for PML-RARA, AML1-ETO, CBFB - MYH11, BCR-ABL, FLT3 [ITD & TKD (D835)], NPM1, and KIT gene mutations were negative. She was started on the 7+3 regimen of AML induction chemotherapy. During the stay, she was supported with multiple pRBC and platelet transfusions. She was shifted to the intensive care unit (ICU) on Day 12 due to repeated hypoglycemia and respiratory distress. She had multiple organ failure and succumbed on Day 16 of induction chemotherapy. This is a case of a patient diagnosed with B-ALL on multiparametric analysis in December 2015, which responded to therapy and was on maintenance therapy, but 4 years later developed secondary AML showing all features consistent with the diagnosis of AML. However, at this time, she showed a complex karyotype with many structural abnormalities.
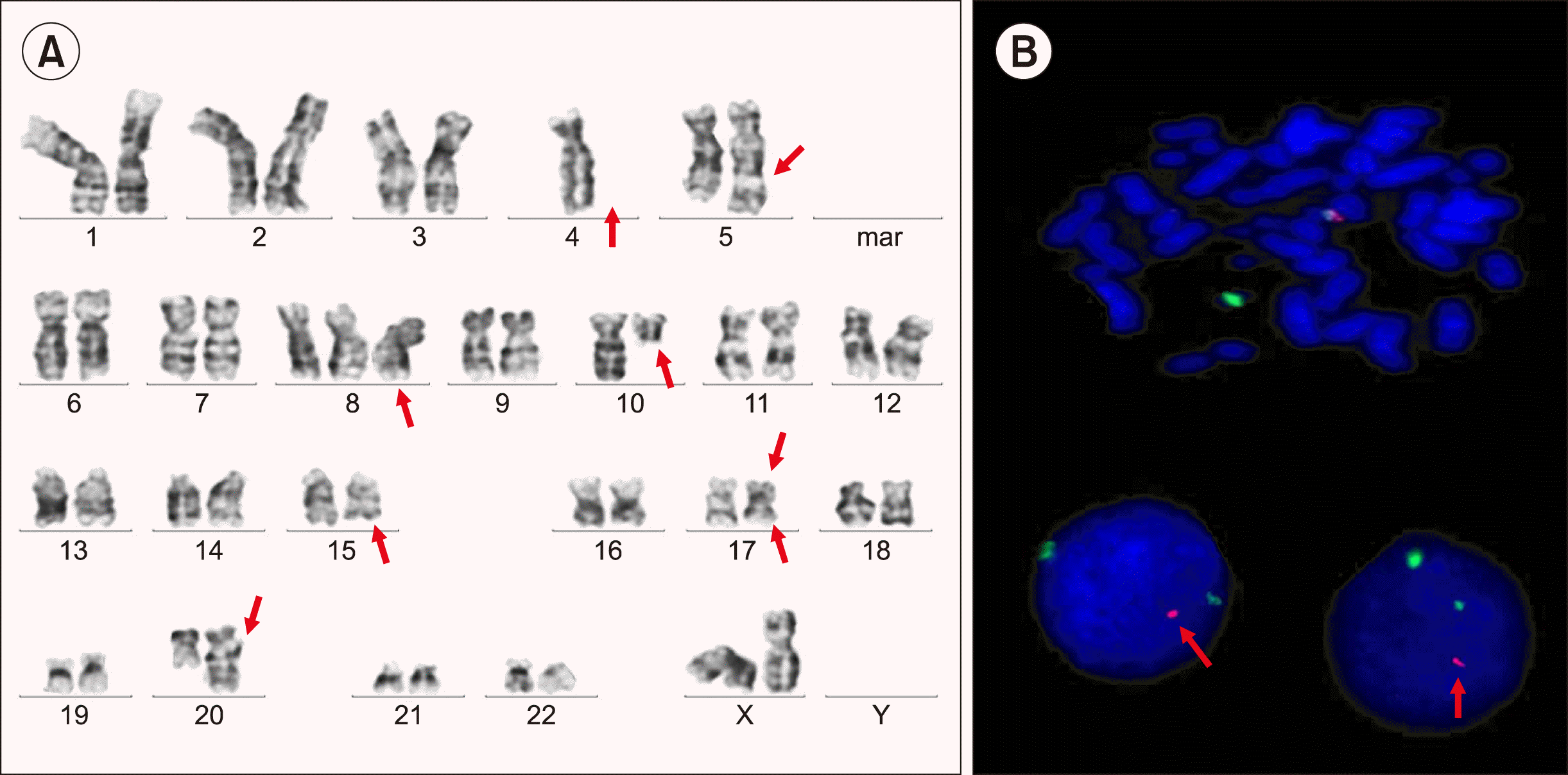
Secondary leukemia with morphologic and immuno-phenotypic features distinct from the primary leukemia following chemotherapy may be secondary to chemotherapy (therapy-related), which is clonally unrelated to the primary neoplasm or a lineage switch which represent clonal evolution of the primary neoplasm. Genetic analysis in the secondary leukemia is required to diagnose whether the second neoplasm is related to the primary neoplasm or not by comparing cytogenetic and/or molecular abnormalities between the primary and secondary leukemia. Diagnosis of lineage switch requires retention of a genetic signature in the secondary leukemia with the switched phenotype [4]. In our cases, cytogenetic analysis demonstrated a change in cytogenetics between the initial B-ALL and relapsed AML. Therapy-related AML is caused by an acquired somatic mutation in hematopoietic stem cells and progenitor cells induced by cytotoxic chemoradiotherapy. Majority of therapy-related leukemia result from the use of alkylating agents, topoisomerase-II inhibitors, and rarely antimetabolites. Daunorubicin, a topoisomerase-II inhibitor, was used in our patient, which may have induced development of secondary AML. The risk of a secondary neoplasm from ALL in adults is lower than that in children; the majority of cases are AML and MDS, non-Hodgkin’s lymphoma (NHL), or rarely solid malignancies. Pagano et al [5]. reported a median latency of 74 months for the diagnosis of ALL to a secondary hematological neoplasm in adult patients. Tavernier et al. [6] reported that the development of all secondary neoplasms was within 0.5 to 13.8 years (median, 4.5 yr) after the diagnosis of ALL, with an overall cumulative risk of secondary neoplasms being 2.1% at 5 years, 4.9% at 10 years, and 9.4% at 15 years. For hematological neoplasms, they reported that the cumulative risk of a second malignancy was 1.8% at 5 years, 2.2% at 10 years, and 3.3% at 18 years [7]. In our case secondary AML developed after 38 months of ALL diagnosis. Complex karyotype is more common in secondary or therapy-related AML rather than in de novo AML. Monosomy 5, del(5q), and monosomy 7 are are also more common in these patients than de novo AML. In our case, the karyotype showed a highly complex karyotype with monosomy 4 and trisomy 8 and 11. An unbalanced translocation between chromosomes 5q and 11q was seen. There was a deletion in the short arm of chromosome 17 at breakpoint p13, which was confirmed on fluorescence in situ hybridization (FISH) using the TP53 deletion probe. A balanced translocation between long arm of chromosome 15q24 and long arm of chromosome 17q23 was also noted. This translocation is different from t(15;17) of acute promyelocytic leukemia, which was seen at breakpoint 15q24 and 17q21. Another balanced translocation between long arm of chromosome 10 and long arm of chromosome 20 was also identified.
This case highlights an unusual case of secondary AML in an adult patient with B-ALL during maintenance chemotherapy, which evolved from a normal karyotype during ALL to a highly complex karyotype as AML developed. It also highlights the importance of performing immuno-phenotyping at the time of relapse in every case of acute leukemia to identify any change in immunophenotyping or any lineage switch to administer the correct therapy and to analyze minimal residual disease on follow-up.
REFERENCES
1. Verma D, O'Brien S, Thomas D, et al. 2009; Therapy-related acute myelogenous leukemia and myelodysplastic syndrome in patients with acute lymphoblastic leukemia treated with the hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone regimens. Cancer. 115:101–6. DOI: 10.1002/cncr.24005. PMID: 19090005. PMCID: PMC4180242.

2. Morton LM, Dores GM, Tucker MA, et al. 2013; Evolving risk of therapy-related acute myeloid leukemia following cancer chemotherapy among adults in the United States, 1975-2008. Blood. 121:2996–3004. DOI: 10.1182/blood-2012-08-448068. PMID: 23412096. PMCID: PMC3624944.

3. Kayser S, Döhner K, Krauter J, et al. 2011; The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood. 117:2137–45. DOI: 10.1182/blood-2010-08-301713. PMID: 21127174.

4. Wu B, Jug R, Luedke C, et al. 2017; Lineage switch between B-lymphoblastic leukemia and acute myeloid leukemia intermediated by "Occult" myelodysplastic neoplasm: two cases of adult patients with evidence of genomic instability and clonal selection by chemotherapy. Am J Clin Pathol. 148:136–47. DOI: 10.1093/ajcp/aqx055. PMID: 28898985.
5. Pagano L, Annino L, Ferrari A, et al. 1998; Secondary haematological neoplasm after treatment of adult acute lymphoblastic leukemia: analysis of 1170 adult ALL patients enrolled in the GIMEMA trials. Gruppo Italiano Malattie Ematologiche Maligne dell'Adulto. Br J Haematol. 100:669–76. DOI: 10.1046/j.1365-2141.1998.00616.x. PMID: 9531332.
6. Tavernier E, Le QH, de Botton S, et al. 2007; Secondary or concomitant neoplasms among adults diagnosed with acute lymphoblastic leukemia and treated according to the LALA-87 and LALA-94 trials. Cancer. 110:2747–55. DOI: 10.1002/cncr.23097. PMID: 17963265.

7. Grosicki S, Barchnicka A, Bodzenta E, Haus O, Jaśkowiec A. 2013; Secondary acute myeloid leukemia (sAML) immediately after intensive chemotherapy of acute lymphoblastic leukemia (ALL). J Leuk. 1:1000102. DOI: 10.4172/2329-6917.1000102.

Fig. 1
Bone marrow aspirate showed lymphoblasts with scanty agranular cytoplasm (A). Bone marrow biopsy (Haematoxylin and Eosin stain, ×400) with IHC showed positivity for CD10 and PAX5 and negativity for CD33 (B).
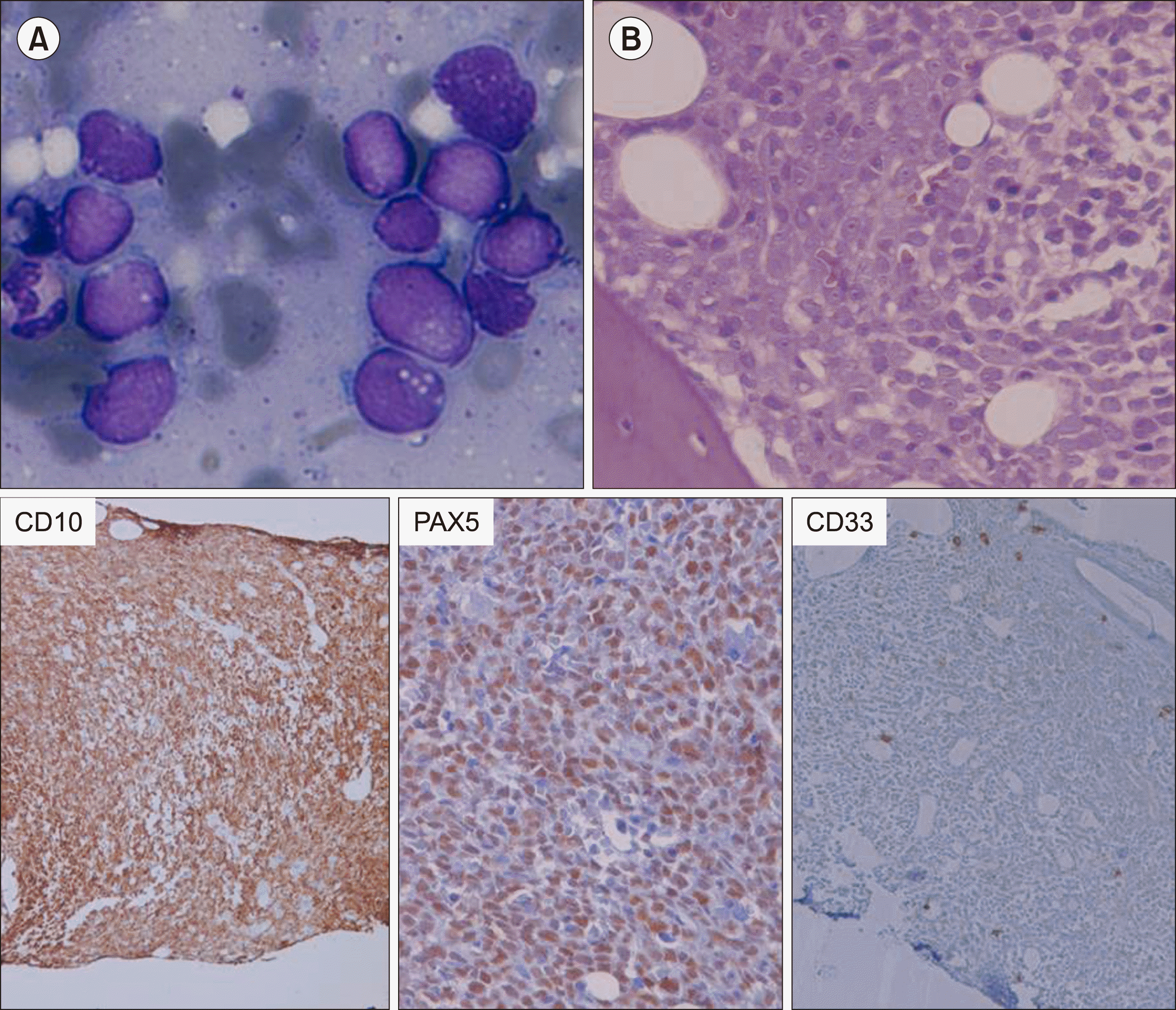
Fig. 2
Bone marrow aspirate showed myeloblasts that were larger in size and had moderate granular cytoplasm (A). Bone marrow biopsy (Haematoxylin and Eosin stain, ×400) with IHC showed positivity for CD33 and negativity for CD10 and PAX5 (B).
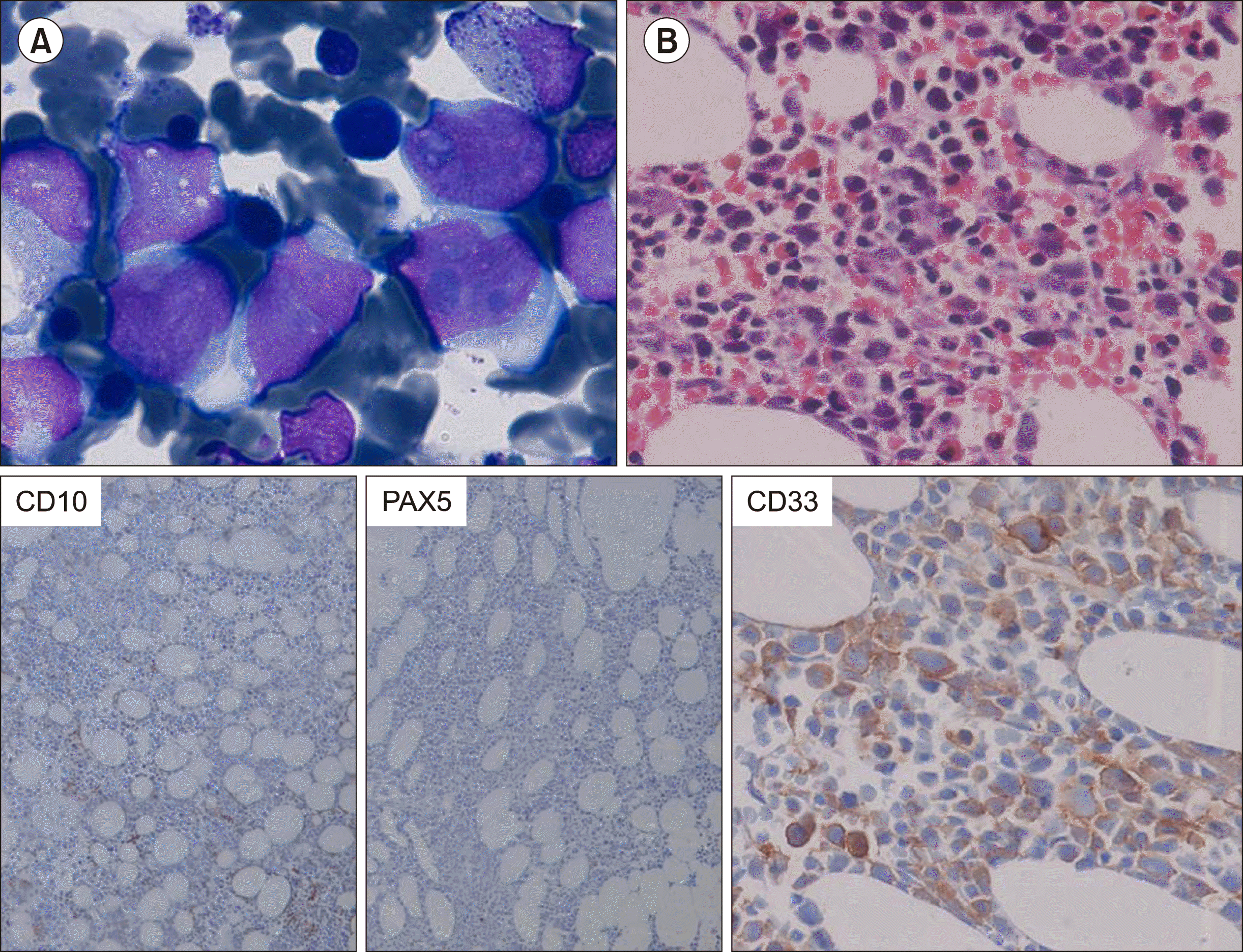
Fig. 3
Karyogram showed 46,XX, -4, +8, der(17) t(15;17)(q24;q23), del(17)(p13), der(20) t(10;20) (q11.2; q13.3), +der(11)t(5;11)(q31;q13), using GTG staining and banding method, ×100 oil immersion, and Carl Zeiss Axioscope Z2, and was processed using IKAROS software (A). Interphase nuclei and metaphase showed monoallelic deletion of TP53 gene localized on 17p13.1 by FISH using LSI 17p13.1(TP53/CEN17) deletion probe [TP53-red, CEN17-green], Zytovysion, Germany (B).




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download