Introduction
Extranodal NK/T cell lymphomas (ENKTCLs) are aggressive cancers that originate from Epstein-Barr virus (EBV) infections. Defects in genes related to antigen presentation and T-cell activation, including human leukocyte antigen (HLA) and programmed cell death-ligand (PD-L) genes, were detected in approximately 30% among other genetic abnormalities. Immune surveillance systems play important roles in viral clearance, lymphomagenesis, and tumor progression. They may serve as prognostic biomarkers and potential targets for immunotherapy. The associations between these immune defects and clinical outcomes, as well as responses to immune checkpoint inhibitors, need to be explored.
Go to : 
Perspective
ENKTCLs are EBV-associated lymphomas with aggressive clinical behaviors. ENKTCL patients typically present with destructive necrotizing tumor masses that affect the upper aerodigestive tract, including the nose, nasopharynx, and paranasal sinuses. Some patients manifest more advanced stages in the lymph nodes, skin, liver, and bone marrow, indicating very poor outcomes. Current treatments include local radiotherapy and combination chemotherapy with L-asparaginase. Immunotherapy and targeted therapy are emerging treatments that may improve patient outcomes, especially in patients with advanced disease [1].
The incidence of ENKTCLs is higher in East Asia and Latin America than in other parts of the world. The genetic background of the HLA system and immune regulation is a possible explanation for the different frequencies among ethnic groups. Two genome-wide association studies conducted in East Asian populations revealed that HLA-DPB1 rs9277378 and rs9271588 and IL18RAP rs13015714 were reported to be strongly associated with ENKTCL susceptibility [2, 3]. Furthermore, a study from Japan reported that ENKTCL risk was also associated with HLA-A*26 and HLA-B*52, which are more prevalent in Asia [4]. These HLA types have been proposed to confer impaired EBV-infected cell eradication by the immune system [5]. The resulting EBV evasion and persistence in NK/T cells may cause neoplastic growth and subsequent ENKTCLs.
EBV infection associated with ENKTCL is classified as a type II latency program because tumor cells usually express EBV-encoded small RNA (EBER), EBV nuclear antigen 1 (EBNA1), and latent membrane protein 1 (LMP1). LMP1 is a viral oncoprotein that can promote abnormal NK/T cell proliferation and may enhance genomic instability via overexpression of activation-induced cytidine deaminase (AID) [4], leading to further oncogenic mutations.
In addition to the HLA system, PD-L1 and PD-L2 play an important role in immune surveillance; however, its role in lymphomagenesis remains unclear. PD-Ls bind to programmed cell death protein (PD) receptors, suppressing cytotoxic T lymphocyte functions, which is an immune escape method used by several cancer types. A high frequency of PD-L1/PD-L2 alterations has been reported among EBV-positive lymphomas, especially ENKTCL, compared to EBV-negative lymphomas (23% vs. 5%) [6]. HLA defects may lead to the dysregulation of the antigen presentation process, whereas PD-L1/PD-L2 overexpression may participate in tumor evasion from cellular immunity corresponding to disease progression. PD-L1/PD-L2 is frequently expressed in ENKTCLs. Notably, patients with PD-L1/PD-L2 expression showed poorer survival compared to patients with low or undetectable PD-L1/PD-L2 expression [7]. Immune checkpoint inhibitors, such as anti-PD1 antibody, are emerging treatments that show promising efficacy for relapsed/refractory ENKTCLs [8, 9]. Therefore, overexpression of PD-L1/PD-L2 is a potential target for immune checkpoint blockade therapy (Fig. 1).
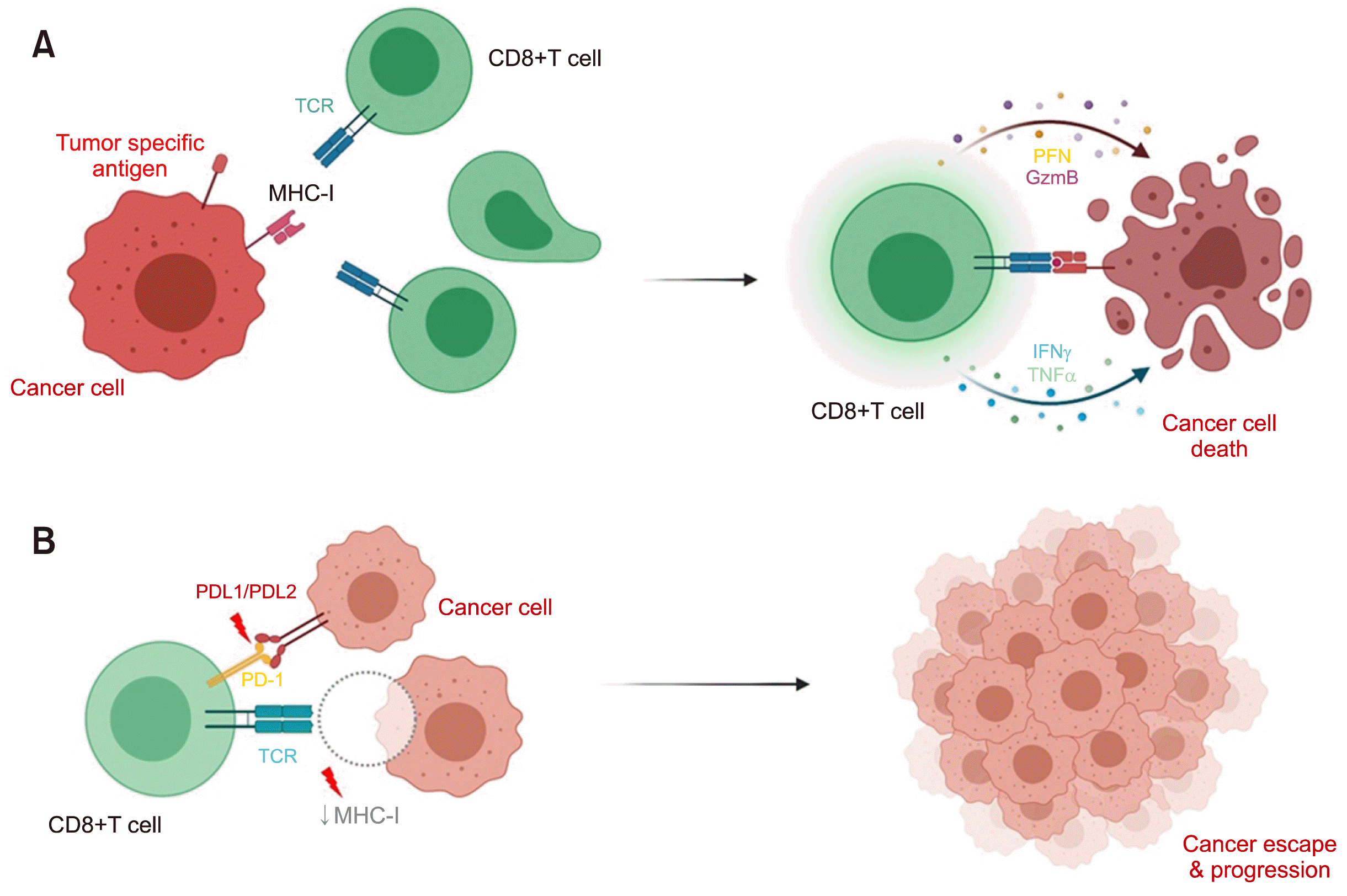 | Fig. 1Immune system and cancer surveillance. In normal situations, CD8+ T-cell recognizes tumor-specific antigens presented by MHC class I and eradicates tumor cells via cytolytic mediators, such as perforin and granzyme (A). Defects of immune surveillance, including dysfunction of PDL1/PDL2 and MHC class I lead to immune escape and cancer progression (B).
Abbreviations: GzmB, granzyme B; IFNγ, interferon-gamma; MHC-I, major histocompatibility complex class I; PD-1, programmed death 1; PDL1/PDL2, programmed death-ligand 1/programmed death-ligand 2; PFN, perforin; TCR, T-cell receptor; TNFα, tumor necrosis factor alpha.
|
Using next-generation sequencing technology, genetic alterations in ENKTCLs were shown to involve various pathways, including epigenetic regulators (58%), RNA helicases (21%), Janus kinase-signal transducer and activator of transcription (JAK-STAT) (26%), other signal transductions (11%), tyrosine phosphatases (26%), tumor suppressors (11%), and immune surveillance (32%) [10-12]. In our study, whole-exome sequencing also revealed abnormalities in ENKTCLs involving HLAs and related genes, which were somatic mutations and copy number variation, accounting for 32% [10]. Loss-of-function mutations in HLA-A were identified in 16% of the cases. Immunohistochemistry analysis revealed that a low HLA expression was associated with advanced stages.
In summary, defective viral antigen presentation may promote lymphomagenesis and progression. Additionally, the PD/PD-L pathway is employed by tumor cells to evade the immune system and can serve as a molecular target for therapy. Studies involving larger numbers of patients with ENKTCL are required to correlate the HLA and PD/PD-L defects with clinical outcomes and clinical responses to anti-PD1 treatments.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download