Abstract
Background/Aims
Fever is a common symptom of acute pancreatitis (AP). This study examined the factors associated with fever due to pancreatic inflammation in the early stages of non-biliary AP.
Methods
This study analyzed the AP database from Kangwon National University Hospital from January 2018 until April 2021 and identified patients who developed fever within 1 week of hospitalization. Patients with gallstone pancreatitis, pseudocyst, walled-off necrosis, chronic pancreatitis, bacteremia, and other site infections were excluded. The febrile group was compared with the afebrile group.
Results
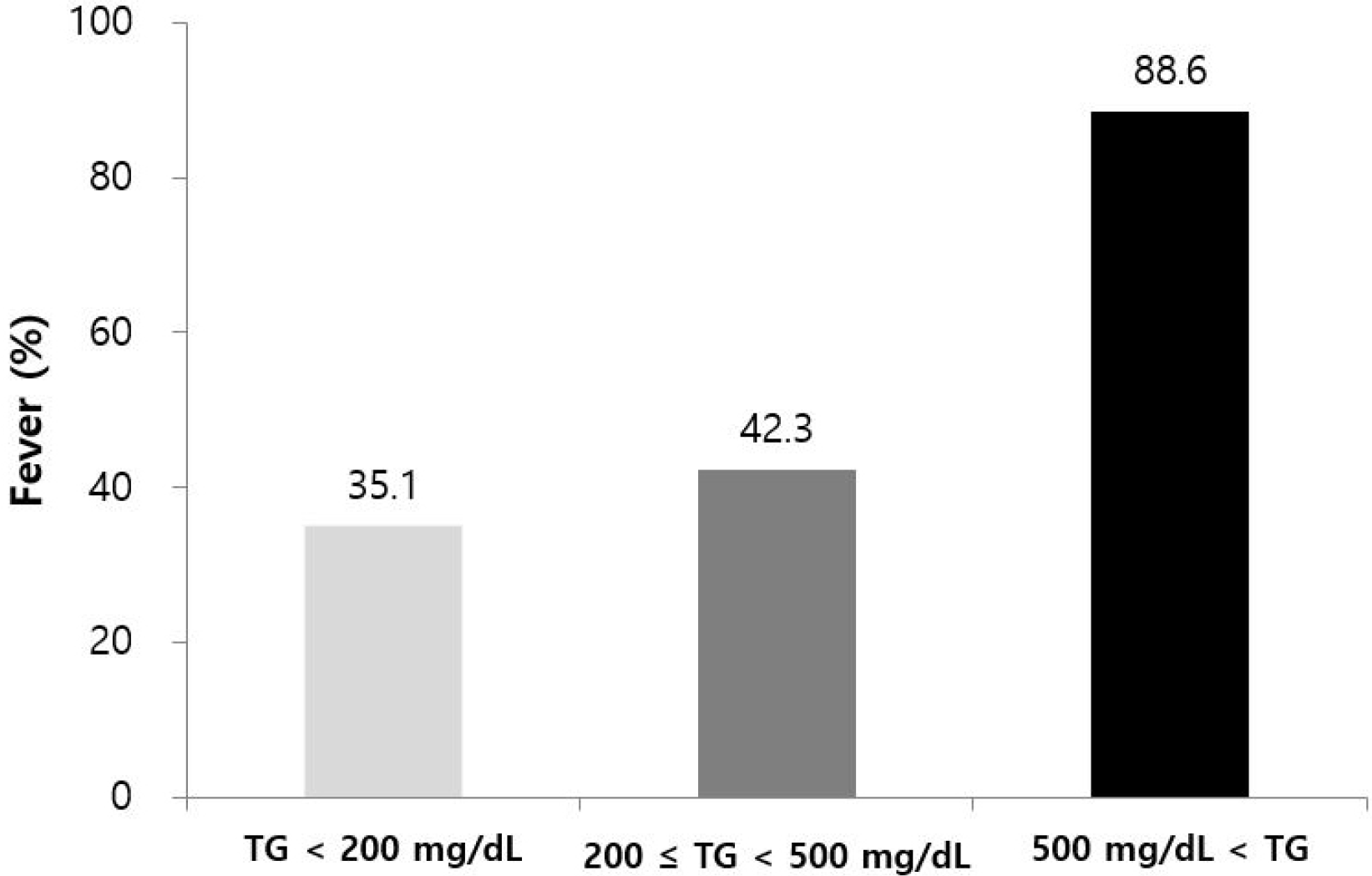
One hundred and fifty-two patients were analyzed, and fever was diagnosed in 79 patients (52.0%). Based on multivariate analysis, fever was positively correlated with hypertriglyceridemia-induced AP (OR 16.8, 95% CI 4.0-70.7, p<0.01) and computed tomography severity index (OR 1.7, 95% CI 1.2-2.6, p<0.01). Recurrent AP was negatively associated with fever (OR 0.3, 95% CI 0.1-0.8, p=0.01). Fever was more frequent in patients with higher initial serum triglyceride (TG) levels than those with lower levels (TG <200 mg/dL; 35.1%, 200≤TG<500 mg/dL; 42.3%, TG ≥500 mg/dL; 88.6%, p<0.01).
Go to : 
Acute pancreatitis (AP) is one of the most common reasons for the hospitalization of patients with gastrointestinal ailments. Previous studies have reported a worldwide increase in the incidence of AP, generally in the range of 20 to 40 per 100,000 individuals.1 AP is one of the leading hospital discharge diagnoses in the United States.2 AP is an acute inflammatory process involving the pancreas. The inflammation may spread to nearby tissues, and lead to a systemic inflammatory response.3 Therefore, fever, leukocytosis, and elevated CRP levels are detected frequently in AP.
There is no evidence to support the role of antibiotics to manage AP successfully, and pancreatic infection is rarely reported in the early phases of AP.4 Therefore, most recent guidelines do not recommend prophylactic antibiotic treatment.5 Despite this, many clinicians still administer antibiotics to patients with AP. In a global study, the rate of antibiotic use has exceeded 80.0% in some regions.6 Fever is one of the main reasons for the antibiotic treatment.7 Therefore, it is important to understand fever induced by pancreatitis in the absence of bacterial infection. This study examined fever-related factors in patients diagnosed with early non-biliary AP.
Go to : 
The AP database of Kangwon National University Hospital, to which patient data were consecutively added and maintained prospectively, was analyzed retrospectively between January 2018 and April 2021. Only patients older than 18 years were enrolled in the database. Alcohol as an etiological factor was determined by the treating physician based on a history of heavy alcohol consumption. However, no specific cutoff or duration of alcohol consumption was defined for the study. The diagnosis of hypertriglyceridemia-induced acute pancreatitis (HTG-AP) was based on the serum triglyceride (TG) levels measured within 48 hours from the initial presentation. The HTG etiology was confirmed by serum TG levels ≥1,000 mg/dL or >500 mg/dL and from the exclusion of other common etiologies by the clinician in a differential diagnosis.8 When the serum TG level was 1,000 mg/dL or higher, HTG was considered the cause of pancreatitis regardless of alcohol consumption. The patient was diagnosed with alcoholic pancreatitis if a patient had a history of excessive alcohol drinking, and the serum TG level was greater than 500 mg/dL and less than 1,000 mg/dL. Biliary pancreatitis was diagnosed when choledocholithiasis was observed in an imaging study, or an abnormality of the liver function test was accompanied by gallbladder stones. If the etiology was unclear, EUS or MRCP was performed to differentiate small stones or tumors.
Patients in the database who were diagnosed with non-biliary AP were identified. Patients with fever (body temperature≥38℃) within a week of hospitalization (febrile group) were compared with the other patients (afebrile group) during the same period. Acute biliary pancreatitis was excluded because it is accompanied frequently by cholangitis. The diagnosis of concurrent cholangitis may be difficult in patients with manifestations of inflammation caused by AP. Prophylactic antibiotics were not used. On the other hand, antibiotics were administered if the treating physician determined that it was necessary. Blood culture of all patients with fever was performed. Patients with bacteremia or other site infections were excluded. The presence of a pseudocyst or walled-off necrosis was another exclusion criterion because it is generally detected in the late phase of AP. Patients with symptoms persisting for more than 1 week before the initial visit or those with a history of AP within 3 months were also excluded. AP combined with chronic pancreatitis was also excluded from the analysis.
The severity of AP was classified as mild, moderately severe, or severe according to the revised Atlanta classification.4 The bedside index of severity in acute pancreatitis (BISAP) was used as an additional scoring parameter. The categorical variables are presented as the frequency and percentage, and the continuous variables are expressed as the mean±SD. A chi-square test or Fisher’s exact test was used to compare the categorical variables. A Student’s t-test was used to compare the continuous variables. Factors with a p-value ≤0.20 in univariate analysis were included in multivariate analysis. The ORs and CIs for fever were calculated by multivariable logistic regression analyses. The statistical significance was established by p<0.05. All statistical analyses were performed using SPSS software, version 26 (IBM, New York, NY, USA). The Institutional Review Board (IRB) of Kangwon National University Hospital approved the study protocol (IRB No. KNUH-2021-08-027).
Go to : 
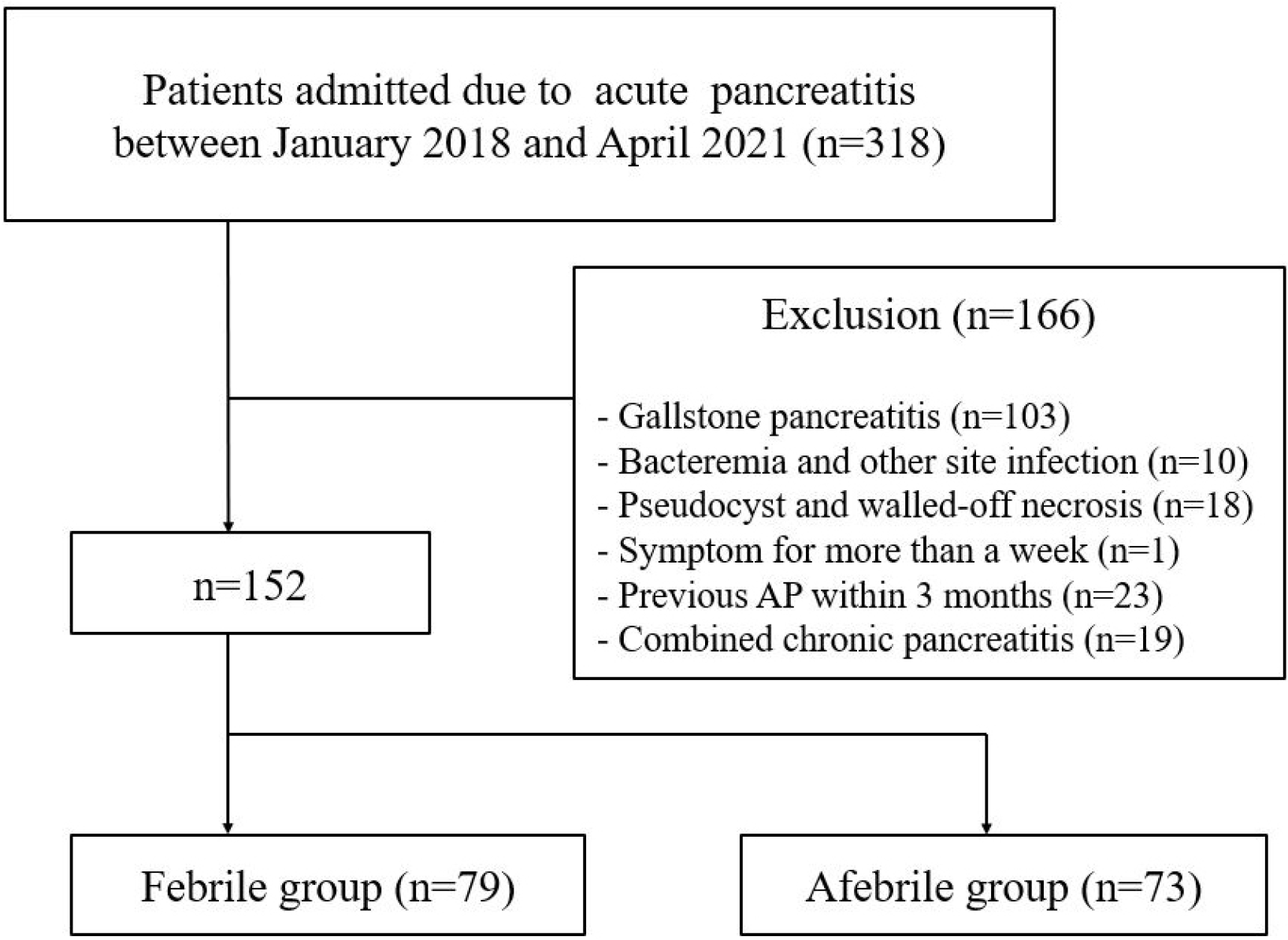
Three hundred and eighteen patients were admitted due to AP, and 166 patients were excluded for the following reasons: gallstone pancreatitis (n=103), bacteremia and other site infections (n=10), pseudocysts and walled-off necrosis (n=18), symptoms persisting for more than a week before initial visit (n=1), previous AP within 3 months (n=23) and combined chronic pancreatitis (n=19). Thus, 152 patients were enrolled in this study (Fig. 1). The mean age was 45.1±15.1 years, and 114 of the 152 patients (75.0%) were male. The serum TG levels were available in 144 patients (94.7%). A history of hypertension was observed in 40 patients (26.3%), and 31 (20.4%) had a history of diabetes mellitus. The most common cause was alcohol (n=85, 55.9%), followed by hypertriglyceridemia (n=33, 21.7%) and others (n=10, 6.6%). Other etiologies included tumor, drugs, hyper-calcemia, and postoperative pancreatic duct strictures. The etiology was unknown in 24 patients (15.8%).
Fever was detected in 79 of the 152 patients (52.0%). HTG-AP was more frequent in the febrile group (36.7%) than in the afebrile group (5.5%, p<0.01). The white blood cell count, total bilirubin, CRP, and TG levels, and the CT severity index (CTSI) scores were higher in the febrile group than in the afebrile group (p<0.01). Table 1 lists the baseline characteristics.
Clinical Characteristics of Patients with or without Fever
Moderately severe to severe AP was more common in the febrile group than in the afebrile group (43.0 vs. 16.4%, p<0.01). The BISAP score was higher in the febrile group than in the afebrile group (1.1 vs. 0.5, p<0.01). The incidence of organ failure (7.6% vs. 2.7%, p=0.28), intensive care unit admission (3.8% vs. 2.7%, p>0.99) and mortality rate (2.5% vs. 1.4%, p>0.99) were similar in both groups (Table 2). There was no statistical difference in the incidence of local complications between the two groups (pseudocyst or walled-off necrosis, 7.6% vs. 2.7%, p=0.28) and recurrence of AP (16.5% vs. 15.1%, p=0.82).
Severity and Mortality of Acute Pancreatitis
Multivariate analyses were used to identify the factors associated with fever. The alcohol and TG levels were excluded before analysis because of the multicollinearity with the etiology (alcoholic pancreatitis and HTG-AP). Multivariate analysis showed that HTG-AP (OR 16.8, 95% CI 4.0-70.7, p<0.01) and CTSI (OR 1.7, 95% CI 1.2-2.6, p<0.01) were factors independently associated with fever. On the other hand, recurrent AP was negatively associated with fever (OR 0.3, 95% CI 0.1-0.8, p=0.01). Smoking, BMI, white blood cell, platelets, total bilirubin, and CRP level did not show a significant difference (Table 3).
Risk Factors Associated with Fever Based on Multivariate Logistic Regression Analysis
Fever was more frequent in patients with higher initial levels of serum TG (TG <200 mg/dL; 35.1%, 200≤TG<500 mg/dL; 42.3%, 500≤TG; 88.6%, p<0.01) (Fig. 2).
Antimicrobial agents had been administered to 46 out of 79 patients (58.2%), and two of them died due to multiorgan failure. No mortality occurred in patients not exposed to antibiotics (41.8%).
Go to : 
Epidemiologic data on AP have rarely been reported in South Korea. In a single-center study, men accounted for two-thirds of all patients. Alcohol was the most common cause, which constituted 59.5% of all cases, followed by gallstones. The recurrent attack rate was 24.5%. The mean age of the patients was nearly 10 years older than in the present study (52.9 years for males and 56.8 years for females).9 A difference in the mean age of the patients was observed between their study9 and the present study. On the other hand, the mean age was similar except for gallstone pancreatitis, which is the most common form of AP in older patients.10,11
Fever is a common symptom of AP. In a previous study, 60% of patients developed fever during the course of AP.12 Cases of gallstone pancreatitis and combined infection were excluded from this study, but the incidence of fever (52.0%) was similar to that of a previous study. In the present study, blood culture was performed in all patients with fever, and patients with bacteremia were excluded from the analysis. Patients whose symptoms developed more than a week ago or those with a history of AP within three months, pseudocyst, or walled-off necrosis were excluded. The study only included cases of early AP. In addition, pancreatic infection is rare in the early phase of AP.4 Therefore, fever diagnosed in the enrolled patients was attributed to pancreatitis alone rather than combined infection.
Based on multivariate analyses, fever was positively correlated with HTG-AP and CTSI. On the other hand, recurrent AP was negatively associated with fever. HTG is a relatively common cause of AP, representing 1-10% of all causes. Although the pathophysiology remains unclear, the formation of free fatty acids may injure the acinar cells and pancreatic capillaries, leading to ischemia and acidosis and resulting in inflammation.13 Because fever is a typical sign of inflammation, the increased prevalence of fever in HTG-AP may reflect the possibility of triglyceride-induced continuous inflammation in early AP. A recent study also reported that systemic inflammatory response syndrome is more common in the HTG-AP group upon admission.14
Brown adipose tissue may also be involved in fever in HTG-AP. Mitochondrial oxidation in brown fat is a significant source of thermogenesis, which increases the body temperature during the febrile response in inflammation.15 Brown fat generates heat upon burning TGs stored within the intracellular lipid droplets, which are replenished by fatty acid absorption from the plasma.16 Therefore, hypertriglyceridemia may fuel heat production by brown adipose tissue.
A recent study reported that HTG aggravates the severity of AP and related complications dose-dependently, even in patients without HTG etiology.17 In the present study, fever was more frequent in patients with higher initial serum TG levels (Fig. 2).
CTSI reflects the degree of pancreatic necrosis, inflammation, and peripancreatic fluid collections. This correlates with complications involving AP.18 A higher score indicates severe and widespread inflammation of the pancreas and surrounding tissue. The lipolysis of peripancreatic fat by activated pancreatic enzymes releases fatty acids and other lipids that act as pro-inflammatory mediators,19 inducing fever. Recurrent AP was less common in the febrile group than in the afebrile group. It is unclear why fever is more frequent during the first attack of AP and whether the clinical manifestations differ in AP with and without relapse.
Prophylactic antibiotics are not recommended, regardless of the severity of AP.5 Although antibiotics are indicated in patients with infected necrosis, no consensus is available regarding the use of antibiotics in other cases. According to global studies, the use of antibiotics in AP varied between 30.0% and 95.0% depending on the region.6,20 On the other hand, there was no association between the rate of antibiotic use and the clinical outcome of AP, suggesting that antibiotics are overused in many countries.6 Indeed, clinical studies reported that the misuse of antibiotics is common in AP, primarily because it is difficult to distinguish between infection and systemic inflammatory response involving pancreatitis at the bedside.20 Fever is the major sign of infection and systemic inflammation. In a survey, a raised body temperature was one of the main reasons warranting antibiotic therapy in AP.21 On the other hand, fever is just one of several findings of systemic inflammation, such as leukocytosis and CRP elevation. A higher white blood cell count or CRP level was not associated with infection in the early phase of AP.6
This study showed that half of all patients developed fever in early non-biliary AP. Antibiotics were not administered in approximately 50.0% of the febrile group, which improved without adverse outcomes. To the best of the authors’ knowledge, this is the first study to examine factors associated with fever in early AP. A better understanding of the risk factors for fever may reduce unnecessary antibiotic use in AP. In patients with early, first-attack HTG-AP, if the CTSI scores are high, the possibility of fever due to pancreatitis appears to be high. In these patients, caution should be used when initiating antibiotics. Of course, antibiotics should be administered without delay in patients with obvious infections, such as bacteremia or air bubbles in necrosis. Although two patients who received antibiotics expired in this study, it is unclear if antibiotics worsened the prognosis of AP because this was not a randomized study. Moreover, there may have been a difference in the severity of pancreatitis between the antibiotic-treated and non-treated groups.
The present study had some limitations. Although antibiotics were not used in many febrile patients, the rate of antibiotic therapy was still high. On the other hand, it was substantially lower than the previously reported rate of antibiotic therapy in South Korea (89.3%).6 In the present study, even patients who were prescribed antibiotics only once were classified as those who received antibiotics. Second, the assignment of the alcoholic etiology was not based on a specific amount or duration of alcohol intake, but it is difficult to measure the amount of alcohol consumed accurately. Moreover, there is no consensus regarding the specific amounts required to induce AP. In addition, the frequency of AP is similar to those reported elsewhere. Finally, the number of patients was small, and all patients were from a single institution. Therefore, these findings might not be generalizable to the broader population. Further studies will be needed to corroborate these findings.
In conclusion, hypertriglyceridemia and CTSI are associated with fever in early non-biliary AP. The pathophysiology of AP can be understood better by analyzing fever, the main symptom of AP, to reduce unnecessary antibiotic use.
Go to : 
Notes
Financial support
The study was supported by 2020 Research Grant from Kangwon National University (D1001811-01-01).
Go to : 
REFERENCES
1. Roberts SE, Morrison-Rees S, John A, Williams JG, Brown TH, Samuel DG. 2017; The incidence and aetiology of acute pancreatitis across Europe. Pancreatology. 17:155–165. DOI: 10.1016/j.pan.2017.01.005. PMID: 28159463.

2. Wadhwa V, Patwardhan S, Garg SK, Jobanputra Y, Lopez R, Sanaka MR. 2017; Health care utilization and costs associated with acute pancreatitis. Pancreas. 46:410–415. DOI: 10.1097/MPA.0000000000000755. PMID: 28099261.

3. Park JM, Shin SP, Cho SK, et al. 2020; Triglyceride and glucose (TyG) index is an effective biomarker to identify severe acute pancreatitis. Pancreatology. 20:1587–1591. DOI: 10.1016/j.pan.2020.09.018. PMID: 33008750.

4. Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013; 62:102–111. DOI: 10.1136/gutjnl-2012-302779. PMID: 23100216.

5. Crockett SD, Wani S, Gardner TB, Falck-Ytter Y, Barkun AN. American Gastroenterological Association Institute Clinical Guidelines Committee. 2018; American Gastroenterological Association Institute guideline on initial management of acute pancreatitis. Gastroenterology. 154:1096–1101. DOI: 10.1053/j.gastro.2018.01.032. PMID: 29409760.
6. Párniczky A, Lantos T, Tóth EM, et al. 2019; Antibiotic therapy in acute pancreatitis: from global overuse to evidence based recommendations. Pancreatology. 19:488–499. DOI: 10.1016/j.pan.2019.04.003. PMID: 31068256.
7. Barrie J, Jamdar S, Smith N, McPherson SJ, Siriwardena AK, O'Reilly DA. 2018; Mis-use of antibiotics in acute pancreatitis: insights from the United Kingdom's National Confidential Enquiry into patient outcome and death (NCEPOD) survey of acute pancreatitis. Pancreatology. 18:721–726. DOI: 10.1016/j.pan.2018.05.485. PMID: 30075909.

8. Scherer J, Singh VP, Pitchumoni CS, Yadav D. 2014; Issues in hypertriglyceridemic pancreatitis: an update. J Clin Gastroenterol. 48:195–203. DOI: 10.1097/01.mcg.0000436438.60145.5a. PMID: 24172179. PMCID: PMC3939000.
9. Youn GJ, Chung WC, Lee JM, Paik CN, Oh JH, Jung SH. 2017; The etiologic evaluation of acute pancreatitis in a general hospital of Seoul-Gyeonggi Province in Korea. Korean J Gastroenterol. 70:190–197. DOI: 10.4166/kjg.2017.70.4.190. PMID: 29060957.

10. Lowenfels AB, Maisonneuve P, Sullivan T. 2009; The changing character of acute pancreatitis: epidemiology, etiology, and prognosis. Curr Gastroenterol Rep. 11:97–103. DOI: 10.1007/s11894-009-0016-4. PMID: 19281696.

11. Yadav D, Lowenfels AB. 2006; Trends in the epidemiology of the first attack of acute pancreatitis: a systematic review. Pancreas. 33:323–330. DOI: 10.1097/01.mpa.0000236733.31617.52. PMID: 17079934.
12. Bohidar NP, Garg PK, Khanna S, Tandon RK. 2003; Incidence, etiology, and impact of Fever in patients with acute pancreatitis. Pancreatology. 3:9–13. DOI: 10.1159/000069146. PMID: 12649559.

13. Pothoulakis I, Paragomi P, Archibugi L, et al. 2020; Clinical features of hypertriglyceridemia-induced acute pancreatitis in an international, multicenter, prospective cohort (APPRENTICE consortium). Pancreatology. 20:325–330. DOI: 10.1016/j.pan.2020.02.010. PMID: 32107193.

14. Kim SJ, Kang H, Kim EJ, Kim YS, Cho JH. 2020; Clinical features and outcomes of hypertriglyceridemia-induced acute pancreatitis: propensity score matching analysis from a prospective acute pancreatitis registry. Pancreatology. 20:617–621. DOI: 10.1016/j.pan.2020.03.013. PMID: 32265135.

15. Morrison SF, Madden CJ, Tupone D. 2012; Central control of brown adipose tissue thermogenesis. Front Endocrinol (Lausanne). 3:5. DOI: 10.3389/fendo.2012.00005. PMID: 22389645. PMCID: PMC3292175.

16. Khedoe PP, Hoeke G, Kooijman S, et al. 2015; Brown adipose tissue takes up plasma triglycerides mostly after lipolysis. J Lipid Res. 56:51–59. DOI: 10.1194/jlr.M052746. PMID: 25351615. PMCID: PMC4274071.

17. Mosztbacher D, Hanák L, Farkas N, et al. 2020; Hypertriglyceridemia-induced acute pancreatitis: a prospective, multicenter, international cohort analysis of 716 acute pancreatitis cases. Pancreatology. 20:608–616. DOI: 10.1016/j.pan.2020.03.018. PMID: 32402696.

18. Simchuk EJ, Traverso LW, Nukui Y, Kozarek RA. 2000; Computed tomography severity index is a predictor of outcomes for severe pancreatitis. Am J Surg. 179:352–355. DOI: 10.1016/S0002-9610(00)00375-5. PMID: 10930478.

19. Noel P, Patel K, Durgampudi C, et al. 2016; Peripancreatic fat necrosis worsens acute pancreatitis independent of pancreatic necrosis via unsaturated fatty acids increased in human pancreatic necrosis collections. Gut. 65:100–111. DOI: 10.1136/gutjnl-2014-308043. PMID: 25500204. PMCID: PMC4869971.

20. Baltatzis M, Jegatheeswaran S, O'Reilly DA, Siriwardena AK. 2016; Antibiotic use in acute pancreatitis: global overview of compliance with international guidelines. Pancreatology. 16:189–193. DOI: 10.1016/j.pan.2015.12.179. PMID: 26804006.

21. Baltatzis M, Mason JM, Chandrabalan V, et al. 2016; Antibiotic use in acute pancreatitis: an audit of current practice in a tertiary centre. Pancreatology. 16:946–951. DOI: 10.1016/j.pan.2016.08.012. PMID: 27613614.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download