Abstract
Background/Aims
There have been few multicenter studies on colonic polyps conducted by primary medical institutions. This study examined the detection rate of colonic polyps in primary health care institutions and the related factors while following the guidelines.
Methods
The medical records of 14,029 patients who underwent colonoscopy between January-June 2020 at 40 primary medical institutions in Korea were analyzed. High-risk adenoma was defined as advanced adenoma, carcinoma, or ≥3 adenomas.
Results
Most patients (71.2%) aged ≥50 years underwent re-colonoscopy within 5 years (51.3%) for diagnostic purposes (61.3%) in Korean primary medical institutions. The detection rates of colon polyps, adenoma, advanced adenoma, high-risk adenoma, and carcinoma was 59.9%, 38.9%, 5.9%, 11.4%, and 0.3% in all subjects and 59.8%, 37.5%, 8.5%, 12.9%, and 0.3% in average-risk patients, respectively. The incidences of adenoma in average-risk patients increased significantly with age (30s/40s/50s: 20.1%/29.4%/43% for adenoma, 4.4%/6.7%/10.3% for advanced adenoma, and 5.6%/9.5%/14.6% for high-risk adenoma; p<0.05). Before 50 years of age, high-risk adenoma was detected in 9.1% of patients in the first-time screening group, and the significant risk factors were being male and ≥40 years of age. The detection rate of high-risk adenoma in the normal index colonoscopy group within 5 years was 9.0%. The significant risk factors included older age, male sex, positive fecal occult blood test, stool form changes, and nonspecific symptoms (gas and indigestion).
Colon cancer is the leading cause of cancer-related mortality worldwide,1 ranking third in cancer-related mortality in 2018 and second in the cancer incidence in 2017 in Korea.2 The incidence of colon cancer and mortality associated with colon cancer can be reduced by early detection and colon polyp removal because most colon cancers occur according to adenoma-carcinoma sequence theory.3 Colonoscopy is the main screening method for detecting colon cancer and conducting follow-up examinations after a colon polyp resection. Guidelines have been suggested, modified, and supplemented continuously according to the research results because it is crucial to ensure that they are suitable for practical situations.4-8 On the other hand, the relevance of generalization is limited in Korea because of the scarcity of studies conducted in primary clinics, with the majority of the studies being conducted in university hospitals. In addition, there are concerns regarding whether treatments are administered according to the guidelines during actual medical practice, e.g., 1) the guidelines recommend the initiation of colonoscopy screening at 50 years of age. If the patient is aged <50 years and asymptomatic with negative fecal occult blood test (FOBT) results, should a colonoscopy be performed as a part of a regular health check-up? 2) should another colonoscopy be performed on patients who have undergone prior high-quality normal colonoscopy <5 years ago? Therefore, this study examined the detection rate of colon polyps in primary medical institutions and provided a solution regarding the treatment being administered according to the guidelines.
This retrospective cross-sectional study involved an analysis of the medical records. From January 2020 to June 2020, 15,813 patients who had undergone a colonoscopy according to the protocol of the Polyp Study at the Korean Society of Digestive Endoscopy (KSDE) at 40 Korean primary medical institutions were selected. Patients aged <20 or ≥90 years, those with colon cancer history and inflammatory bowel disease, and those with an incomplete examination due to poor bowel preparation or difficulty in cecal insertion were excluded. The Public Institutional Bioethics Committee of the Ministry of Health and Welfare reviewed this study (approval No. P01-202009-21-013). The Institutional Review Board (IRB) of Korea National Institute for Bioethics Policy waived the need for informed consent.
Patients’ information, such as sex, age, height, weight, body mass index, diabetes, and colonoscopy performance history (first time, within 5 years, ≥5 years) was retrieved from the medical records. The indications for colonoscopy were identified: being asymptomatic; experiencing abdominal pain, bloody stool, constipation, diarrhea, stool form changes, anemia, weight loss, and nonspecific symptoms (gas, indigestion, and mucoid stool); and demonstrating the presence of a positive FOBT result, prior history of colon polyps (except colorectal cancer), and family history (FHx) of colon cancer (parents and siblings). Multiple entries were allowed in the case of >1 indication.
The total number, size, location, macroscopic shape, and pathology findings of polyps detected during colonoscopy were investigated. Based on the U.S. Multi-Society Task Force guidelines, advanced adenoma was defined as having an adenoma ≥1 cm in size, or with (tubulo)villous histology or high-grade dysplasia, whereas advanced neoplasia was defined as having advanced adenoma or carcinoma.8 Furthermore, high-risk adenoma was defined as having advanced neoplasia or ≥3 adenomas.8
The IBM SPSS Statistics for Windows, version 26 (IBM Corp, Armonk, N.Y., USA) statistical analysis package was used. All p-values were two-sided, and a p-value <0.05 was considered significant. A chi-square test and linear-by-linear association were used to compare the proportions of the categorical variables. The risk factors of high-risk adenoma were determined by performing multiple logistic regression analysis in a forward hierarchical stepwise method to select the independent variables related to high-risk adenoma. All significant variables in univariate analysis were included in the model and were retained at each step if p<0.05.
The medical records of 15,813 patients who underwent a colonoscopy were reviewed. Of them, 1,757 patients were excluded because they were aged <20 years (n=125) or ≥90 years (n=7), a history of colon cancer (n=421) and inflammatory bowel disease (n=433), and incomplete examination attributed to poor bowel preparation or difficulty in cecal insertion (n=771). Ultimately, 14,056 patients were analyzed. Among them, 26 patients with incomplete medical records, such as pathological findings on polyps, were excluded, leaving 14,029 in this study. There were 6,949 males (49.5%) and 7,080 females (50.5%). The mean age was 55.9±13.4 years for males and 57.2±12.7 years for females; 9,986 patients (71.2%) were aged ≥50. Colonoscopy was performed for the first time, within 5 years, and ≥5 years after the initial screening in 26.8%, 51.3%, and 17.4% patients, respectively (Table 1).
Of the 14,029 patients, colon polyp, adenoma, advanced adenoma, high-risk adenoma, and carcinoma were detected in 59.9%, 38.9%, 5.9%, 11.4%, and 0.3% of all the patients, respectively; 59.8%, 37.5%, 8.5%, 12.9%, and 0.3% of the average-risk patients, respectively; 66.8%, 44.0%, 3.9%, 13.3%, and 0.0% of the patients in polyp surveillance, respectively; and 58.1%, 38.1%, 5.4%, 10.6%, and 0.4% of the symptomatic patients, respectively (Table 2). The average-risk patients were divided into two groups based on age. Colon polyps, adenoma, advanced adenoma, and high-risk adenoma were detected in 62.2%, 44.0%, 9.5%, and 15.2% of the patients, respectively, in the ≥50-year-old group, which were significantly higher than those detected in the <50-year-old group (p<0.05). On the other hand, carcinoma was found in 6 patients (0.3%) in the ≥50-year-old group and 5 patients (0.4%) in the <50-year-old group, which was not significant (p=0.765) (Table 3).
The detection rate of colon polyps, adenoma, advanced adenoma, high-risk adenoma, and carcinoma in all the patients increased significantly with age (Fig. 1). In the average-risk patient group, when those in their 30s, 40s, and 50s were compared, the incidence of polyps was 50.8%, 56.6%, and 63.4%, respectively; adenoma was 20.1%, 29.4%, and 43%, respectively; advanced adenoma was 4.4%, 6.7%, and 10.3%, respectively; and high-risk adenoma was 5.6%, 9.5%, and 14.6%, respectively, showing an increasing trend (p<0.05) (Fig. 2).
High-risk adenoma increased with age in males and females aged 20-70. On the other hand, the prevalence of high-risk adenoma was high in men of the same age group except for those in their 20s and 80s. The detection rate of high-risk adenoma in all the patients in their 30s, 40s, 50s, 60s, and 70s was 3.7%, 7.7%, 15.1%, 18.9%, and 23% in males and 2%, 4.4%, 6.5%, 10.7%, and 14.8% in females, respectively (Fig. 3). The detection rate of high-risk adenoma in the average-risk patients in their 30s, 40s, 50s, 60s, and 70s was 7.6%, 11.6%, 21.2%, 23.6%, and 23.9% in males and 2.5%, 6.3%, 9.7%, 10.6%, and 16.1% in females, respectively (Fig. 4). The age group with a similar prevalence in men and women showed a difference of ~10 years among patients in their 40s and 50s and ~20 years among patients in their 60s and 70s.
This study analyzed 725 asymptomatic patients aged <50 years who underwent the initial screening colonoscopy. Among them, advanced adenoma, advanced neoplasia, and high-risk adenoma were detected in 54 (7.4%), 55 (7.6%), and 66 patients (9.1%), respectively. Multivariable logistic regression analysis showed that an age of ≥40 years and male sex were significant risk factors for high-risk adenoma (Table 4).
Of the 14,029 patients, 13,396 whose colonoscopy performance history had been investigated were analyzed. Among them, 7,197 patients (53.7%) had a colonoscopy follow-up period of <5 years, 2,438 (18.2%) had a follow-up period of ≥5 years, and 3,761 (28.1%) underwent screening for the first time. In both the polyp surveillance and normal index colonoscopy groups, the detection rate of high-risk adenoma was significantly higher in the patient group with a follow-up period of ≥5 years than that in the group with <5 years. On the other hand, high-risk adenomas were detected in 727 (10.1%), 248 (12.4%), and 298 patients (9.0%) in the all-patient group, the polyp surveillance group, and the normal index colonoscopy group with a follow-up period of <5 years, respectively (Table 5).
Furthermore, multivariable logistic regression analysis showed that the significant risk factors of high-risk adenoma in the normal index colonoscopy group with a follow-up period of <5 years were advanced age and male sex as well as the presence of a positive FOBT result, stool form changes, and nonspecific symptoms (gas, indigestion) (Table 6).
Most studies conducted on the colon polyps involved single or tertiary medical institutions. Therefore, multicenter studies at primary medical institutions are required to investigate the relevance of generalization. In this study, ~51.3% of the total patients underwent colonoscopy rescreening within five years after the first screening, suggesting that the actual treatment performed in primary medical institutions is considerably different from the guideline recommendations. This is not an issue merely in Korea because similar patterns have also been observed in North America and Europe.9 The main issue regarding following the guidelines in primary medical institutions is that these guidelines are based on the results of high-quality index colonoscopy. In other words, in primary medical institutions where the decision to perform colonoscopy is made without knowing the index colonoscopy findings, colonoscopy is performed more frequently than suggested in the guidelines based on doctors’ personal experience. In a nationwide survey conducted by Hong et al.10, the main reasons for choosing a period earlier than the guidelines included the fear of overlooking cancer, low examination costs in Korea, and suspicions regarding the quality and results of previous colonoscopy.
In this study, most patients (71.2%) aged ≥50 underwent recolonoscopy within 5 years (51.3%) for diagnostic purposes (61.3%) in Korean primary medical institutions. The detection rates in average-risk patients were 37.5% for adenoma, 8.5% for advanced adenoma, 12.9% for high-risk adenoma, and 0.3% for carcinoma. In previous studies, the prevalence of adenoma and advanced adenoma in Korea was 23.9-33.1% and 2.1-7.1%, respectively.11-13 According to the results of the present study, adenoma cases appear to be increasing in Korea. The difference between the adenoma detection rate of this study and other studies may have resulted from the trends of the times and differences in the study design and institution type (i.e., primary clinics vs. tertiary hospitals). Nevertheless, it cannot be said that colonic adenoma cases are increasing in Korea because this study was a retrospective study for only six months, making it difficult to generalize and included data that did not represent the entire population. Therefore, large-scale research would be required to evaluate the prevalence of colonic adenoma accurately. In this study, the rate of adenoma detection in average-risk patients in their 30s and 40s was 20.1% and 29.4%, respectively, and that of advanced adenoma was 4.4% and 6.7%, respectively. In other studies, the prevalence of adenoma was 10.4-13.4% and 22.2-28.1% in patients in their 30s and 40s, respectively, and the prevalence of advanced adenoma was 0.4-0.7% and 2.4-2.7%, respectively. In similar studies, the prevalence increased with age. In particular, the frequency increased in patients in their 40s.12,13 In this study, high-risk adenoma increased with age in both males and females (aged 30-70 years) of the average-risk patient group. On the other hand, the detection rate of high-risk adenoma increased approximately 10–20 years early in men except for those in their 80s. In this study, the rate of high-risk adenoma detection in males decreased, and that in females increased when the patients aged 80 and 70 years were compared. These results might be due to selection bias caused by the very small number of patients aged 80 years (n=37) compared to the other age groups. Another reason may be that compared to the other age groups, the rate of a previous colonoscopy over a 5-year follow-up period was lower in males aged 80 years and higher in females aged 80 years. Therefore, the detection rate of high-risk adenoma was much higher in females aged 80 years. Lieberman mentioned a lag time of ~7-8 years on the risk of colon cancer and the occurrence of advanced polyps between males and females because of the influence of female hormones. Hence, it is necessary to delay the initiation of screening in females more than in males.14 On the other hand, Regula et al.15 reported a difference of approximately 10 years between men and women. They suggested that men should receive screening at an earlier age than women. Park et al.12 suggested that the detection rate of adenoma in men increases from their 40s. Despite this, the results were difficult to generalize because the study was conducted at tertiary medical institutions located mainly in cities.12 In the present study, the rate of high-risk adenoma in the average-risk males aged 40 years was 11.6%. Therefore, more studies will be needed to determine if the detection rate of high-risk adenoma in males aged 40 years is meaningful enough to lower the starting age for colorectal cancer screening.
In this study, advanced adenoma, advanced neoplasia, and high-risk adenoma were found in 7.4%, 7.6%, and 9.1% of the 725 patients who underwent colonoscopy for screening purposes before 50 years of age, respectively. Among them, the significant risk factors for high-risk adenoma were age ≥40 years and male sex. In previous studies, the prevalence of advanced adenoma in the same group was 1.6-3.5% (n=27-53).12,13,16,17 Chung et al.13 reported that the risk factors were male sex, smoking, and FHx of colon cancer. That study was different from the present study because smoking was not investigated, and the FHx of colon cancer was not found to be a significant risk factor in the present study.13 Regula et al.15 reported that the prevalence of advanced neoplasia was 3.4% (n=243), and the risk factor was male sex, which was consistent with the results of this study. The higher detection rate of advanced adenoma and neoplasia in this study than previous studies is related to the recent trend of increasing colorectal cancer found in younger individuals. Therefore, to determine the appropriate starting age for colorectal cancer screening, additional studies on the cost-effectiveness and meaning of the prevalence of advanced adenoma and neoplasia detected in the young population will be needed.
In this study, the prevalence of high-risk adenoma was significantly higher in the group with a follow-up period of ≥5 years than the group with a follow-up period of <5 years (12.3% vs. 10.1%). These characteristics were the same in the polyp surveillance group (19.4% vs. 12.4%) and normal index colonoscopy group (11.1% vs. 9.0%). Therefore, in terms of the risk of colon cancer, regardless of the inaccurate results of index colonoscopy, another colonoscopy should be performed 5 years after the first colonoscopy. In previous studies, the 5-year cumulative incidence of metachronous advanced adenoma was reported to be 2.0-3.3% and 2.4-4.9% in the no adenoma and baseline low-risk adenoma groups, respectively.8,18,19 The reason for the higher rate of high-risk adenoma in this study than other studies may be the analysis, including three or more adenomas, and the possibility of a high-risk population being included because of the inaccurate results of index colonoscopy. Nevertheless, considering that high-risk adenoma was found in 9.0% of the <5-year normal index colonoscopy group, it is difficult not to recommend an additional colonoscopy even if the follow-up period is <5 years. Accordingly, the risk factors of high-risk adenoma were analyzed in the normal index colonoscopy group within 5 years. The significant risk factors were increased age, male sex, positive FOBT result, stool form changes, and nonspecific symptoms (gas and indigestion). Therefore, patients with the abovementioned risk factors may require another colonoscopy despite being early.
This study overcame selection bias, one of the short-comings in studies involving single or tertiary medical institutions, and analyzed target groups with various socioeconomic characteristics, making it easier to generalize the findings. On the other hand, obtaining detailed patient information was not possible because this study was retrospective. In addition, the detection rate or the number of colon polyps may have been underestimated or over-estimated because of the short study period. Therefore, this study cannot represent the prevalence of colonic polyps in all Korean primary medical institutions. Nevertheless, this large-scale multicenter study is significant because it identified the colonoscopy-related status and the detection rate of colon polyps in primary medical institutions throughout the country. In addition, this study identified the detection rate of polyps in colonoscopy performed based on inaccurate results of the index colonoscopy, which is often performed at the actual treatment site, and made suggestions regarding the current medical practice.
In conclusion, this study identified the detection rate of colon polyps in primary medical institutions and presented the considerations for both initial screening and follow-up. Nevertheless, a larger prospective colon polyp study targeting primary medical institutions will be needed. This study may complement the guidelines for colon polyps.
ACKNOWLEDGEMENTS
The authors thank the other members of the KSDE Polyp Study Workgroup who participated in this study.
Seoul SOK Clinic, Seoul, Korea: Chi Wook Song, Yun Bae Kim, and Hwang Rae Chun; Sang-In SOK Clinic, Daegu, Korea: Jin Hyung Park, Young Hak Lee, and Jonghyup Lee; Busan SOK Internal Medicine, Busan, Korea: Hyungha Jang; Ulsan Comfort Gastroenterology Clinic, Ulsan, Korea: Dae Hyun Kim; Hana General Internal Medicine Clinic, Daegu, Korea: KyuHyun Cho; Sunwoosoksiwon Internal Medicine Clinic, Daegu, Korea: Changkeun Woo; Jeon's Internal Medicine, Ulsan, Korea: Jeonghun Kim; Dr. Kim IM, Busan, Korea: Yongtae Kim, Wooseong Kim, and Sung Jun Kim; Insarang Medical & Radiologic Clinic, Busan, Korea: Won Sik Chang; Dr. Cho Medical Clinic, Busan, Korea: Yeong Sam Cho; Seoul Goodmorning Internal Medicine Clinic, Seoul, Korea: Dae Han Choi; You And Jang Internal Medicine Hosp., Daegu, Korea: Hyon Uk Ryu; Woori Internal Medicine Clinic, Daegu, Korea: Junhyuk Son; Yeon San Int Med, Busan, Korea: Chihoon Kim; Yonsei Clinic, Banyo, Busan, Korea: Chan Kyoung Kim; Gyungangjeil Internal Medicine Clinic, Daegu, Korea: Seokjoon Je; BMKD Medical Center, Daegu, Korea: Sejin Hwang; Healthplus Union Clinic, Daegu, Korea: Jong-Hae Pack; Park Kwan Sik Medical Clinic, Busan, Korea: Kwan Sik Park; Kimskeonkang Internal Medicine, Daegu, Korea: Kwang Hyun Kim; Myungin Internal Medicine Clinic, Busan, Korea: Sungmok Kim; Hadan Soksiwon Internal Medicine, Busan, Korea: Jung Nam Lee; Busan Internal Medicine, Busan, Korea: Dong Whan Kahng; Dr. Ok's Internal Medicine Clinic, Busan, Korea: Changmin OK; Charm Zoen Internal Medicine Clinic, Busan, Korea: Yong Mock Bae; Maumsok Internal Medicine Clinic, Incheon, Korea: Byung Youn Hwang; Esia IM Clinic, Daegu, Korea: Changwook Park; Dr. Ham's medical clinic, Incheon, Korea: Jeongsik Ham; Cheongna nanum clinic, Incheon, Korea: Jihun Kim.
REFERENCES
1. Ferlay J, Soerjomataram I, Dikshit R, et al. 2015; Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. DOI: 10.1002/ijc.29210. PMID: 25220842.

2. Annual report on the cause of death statistics, 2018. [Internet]. Seoul (KR): Korea National Statistical Office (KNSO);2020. cited 2020 Jan 29. Available from: https://kosis.kr/publication/publicationThema.do.
3. Winawer SJ, Zauber AG, Ho MN, et al. 1993; Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 329:1977–1981. DOI: 10.1056/NEJM199312303292701. PMID: 8247072.
4. Lee BI, Hong SP, Kim SE, et al. 2012; Korean guidelines for colorectal cancer screening and polyp detection. Clin Endosc. 45:25–43. DOI: 10.5946/ce.2012.45.1.25. PMID: 22741131. PMCID: PMC3363119.

5. Yang DH, Hong SN, Kim YH, et al. 2012; Korean guidelines for postpolypectomy colonoscopy surveillance. Clin Endosc. 45:44–61. DOI: 10.5946/ce.2012.45.1.44. PMID: 22741132. PMCID: PMC3363120.

6. Cairns SR, Scholefield JH, Steele RJ, et al. 2010; Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut. 59:666–689. DOI: 10.1136/gut.2009.179804. PMID: 20427401.

7. Rex DK, Boland CR, Dominitz JA, et al. 2017; Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 86:18–33. DOI: 10.1016/j.gie.2017.04.003. PMID: 28600070.

8. Gupta S, Lieberman D, Anderson JC, et al. 2020; Recommendations for follow-up after colonoscopy and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 158:1131–1153.e5. DOI: 10.1053/j.gastro.2019.10.026. PMID: 32044092. PMCID: PMC7672705.

9. Djinbachian R, Dubé AJ, Durand M, et al. 2019; Adherence to post-polypectomy surveillance guidelines: a systematic review and meta-analysis. Endoscopy. 51:673–683. DOI: 10.1055/a-0865-2082. PMID: 30909308.

10. Hong S, Suh M, Choi KS, et al. 2018; Guideline adherence to colonoscopic surveillance intervals after polypectomy in Korea: results from a nationwide survey. Gut Liver. 12:426–432. DOI: 10.5009/gnl17403. PMID: 29429156. PMCID: PMC6027840.

11. Park DI, Kim YH, Kim HS, et al. 2006; Diagnostic yield of advanced colorectal neoplasia at colonoscopy, according to indications: an investigation from the Korean Association for the Study of Intestinal Diseases (KASID). Endoscopy. 38:449–455. DOI: 10.1055/s-2006-925227. PMID: 16767578.

12. Park HW, Byeon JS, Yang SK, et al. 2009; Colorectal neoplasm in asymptomatic average-risk Koreans: The KASID prospective multicenter colonoscopy survey. Gut Liver. 3:35–40. DOI: 10.5009/gnl.2009.3.1.35. PMID: 20479899. PMCID: PMC2871563.

13. Chung SJ, Kim YS, Yang SY, et al. 2010; Prevalence and risk of colorectal adenoma in asymptomatic Koreans aged 40-49 years undergoing screening colonoscopy. J Gastroenterol Hepatol. 25:519–525. DOI: 10.1111/j.1440-1746.2009.06147.x. PMID: 20370730.
14. Lieberman D. 2010; Progress and challenges in colorectal cancer screening and surveillance. Gastroenterology. 138:2115–2126. DOI: 10.1053/j.gastro.2010.02.006. PMID: 20167216.

15. Regula J, Rupinski M, Kraszewska E, et al. 2006; Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med. 355:1863–1872. DOI: 10.1056/NEJMoa054967. PMID: 17079760.

16. Imperiale TF, Wagner DR, Lin CY, Larkin GN, Rogge JD, Ransohoff DF. 2002; Results of screening colonoscopy among persons 40 to 49 years of age. N Engl J Med. 346:1781–1785. DOI: 10.1056/NEJM200206063462304. PMID: 12050337.

17. Rundle AG, Lebwohl B, Vogel R, Levine S, Neugut AI. 2008; Colonoscopic screening in average-risk individuals ages 40 to 49 vs 50 to 59 years. Gastroenterology. 134:1311–1315. DOI: 10.1053/j.gastro.2008.02.032. PMID: 18471508. PMCID: PMC3673301.

18. Chung SJ, Kim YS, Yang SY, et al. 2011; Five-year risk for advanced colorectal neoplasia after initial colonoscopy according to the baseline risk stratification: a prospective study in 2452 asymptomatic Koreans. Gut. 60:1537–1543. DOI: 10.1136/gut.2010.232876. PMID: 21427200.

19. Lieberman DA, Weiss DG, Harford WV, et al. 2007; Five-year colon surveillance after screening colonoscopy. Gastroenterology. 133:1077–1085. DOI: 10.1053/j.gastro.2007.07.006. PMID: 17698067.

Fig. 1
Detection rate of colon polyps in all the patients according to age group. The detection rate of polyp, adenoma, advanced adenoma, and high-risk adenoma increased with age.
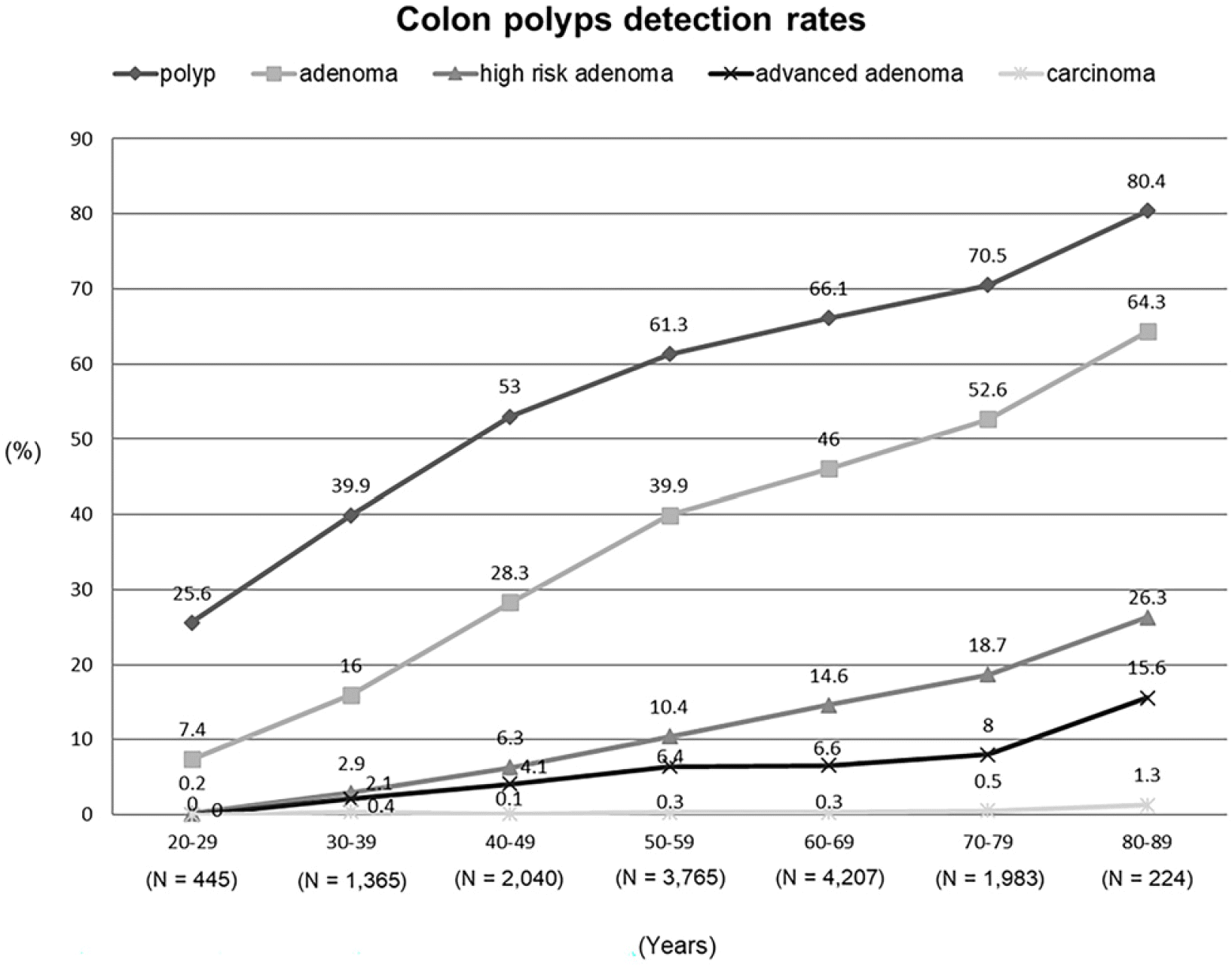
Fig. 2
Detection rate of colon polyps in average-risk patients according to age group. The detection rate of polyp, adenoma, advanced adenoma, and high-risk adenoma increased with age.
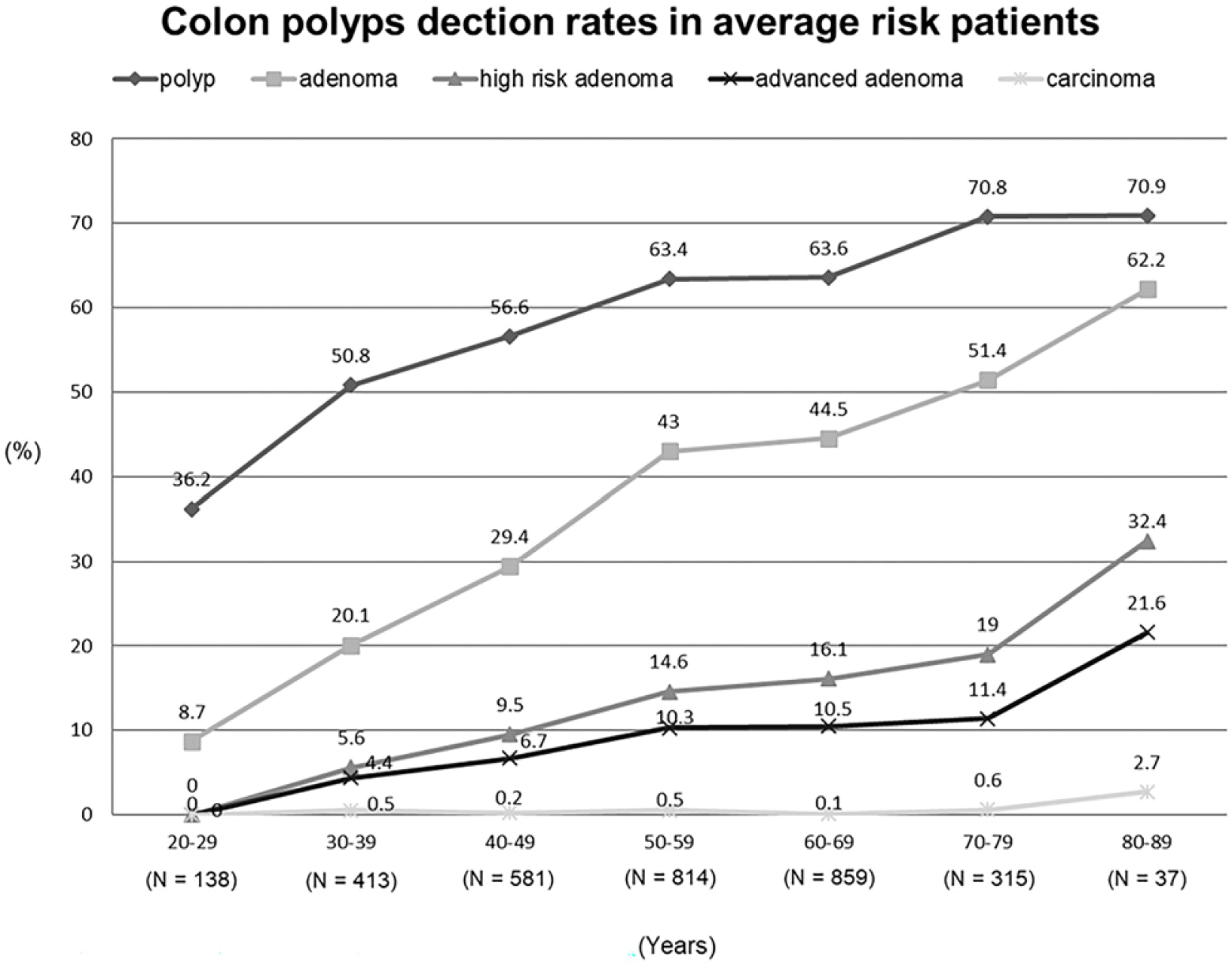
Fig. 3
Detection rate of high-risk adenoma in all the patients according to age group and sex. The detection rate of high-risk adenoma in males was higher than females of the same age group except for those in their 20s and 80s.

Fig. 4
Detection rate of high-risk adenoma in average-risk patients according to age group and sex. The detection rate of high-risk adenoma in males was higher than females of the same age group except for those in their 80s.
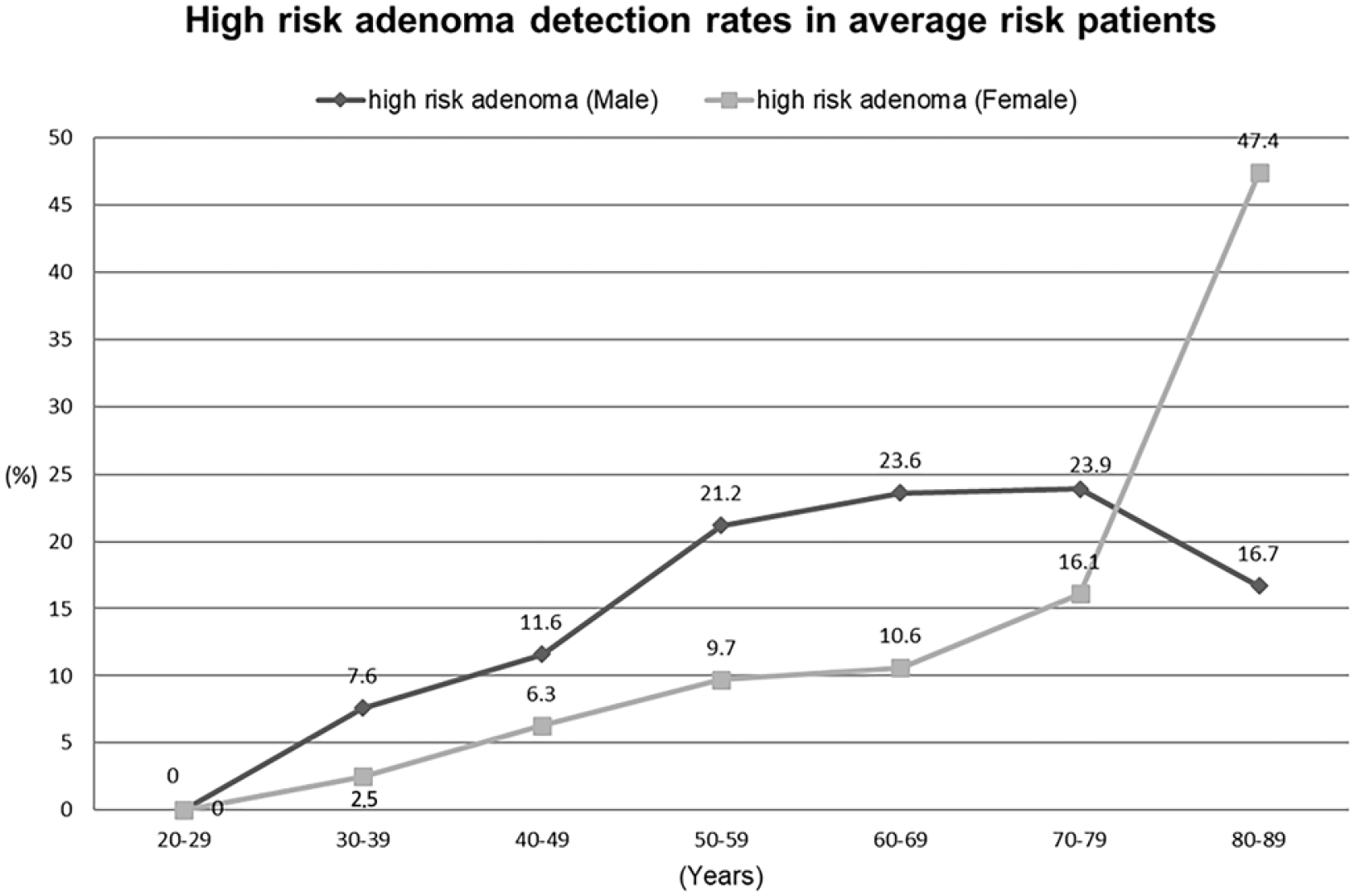
Table 1
Characteristics of the Total 14,029 Patients
Table 2
Detection Rate of Colon Polyps According to Colonoscopy Indication
Table 3
Detection Rate of Colon Polyps in Average-risk Group According to Age
| Variable | Total number of screening cases (n=3,157) | Number of cases aged <50 years (n=1,278) | Number of cases aged ≥50 years (n=1,879) | p-valuea |
|---|---|---|---|---|
| Polyp | 1,889 (59.8) | 721 (56.4) | 1,168 (62.2) | 0.001b |
| Adenoma | 1,183 (37.5) | 357 (27.9) | 826 (44.0) | 0.000b |
| Advanced adenoma | 269 (8.5) | 91 (7.1) | 178 (9.5) | 0.020b |
| High-risk adenoma | 407 (12.9) | 121 (9.5) | 286 (15.2) | 0.000b |
| Carcinoma | 11 (0.3) | 5 (0.4) | 6 (0.3) | 0.765 |
Table 4
Logistic Regression Analysis for the Risk Factors of High-risk Adenoma in Asymptomatic Patients Aged <50 Years Undergoing First Time Colonoscopy
| Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|
|
|
|
||||
| OR | p-value | OR | 95% CI | p-value | |
| Sex | |||||
| Female | 1 (referent) | ||||
| Male | 2.045 | 0.038a | 2.190 | 1.139-4.210 | 0.019a |
| Age (years) | |||||
| Numerical | 1.142 | 0.000a | 1.147 | 1.088-1.209 | 0.000a |
| 20-39 | 1 (referent) | ||||
| 40-49 | 2.858 | 0.001a | 3.912 | 2.218-6.898 | 0.000a |
| DM | |||||
| (-) | 1 (referent) | ||||
| (+) | 1.633 | 0.437 | 1.356 | 0.386-4.764 | 0.634 |
| CRC FHx | |||||
| (-) | 1 (referent) | ||||
| (+) | 1.114 | 0.886 | 1.139 | 0.258-5.032 | 0.863 |
| BMI | |||||
| <25 | 1 (referent) | ||||
| ≥25 | 1.499 | 0.175 | 1.234 | 0.672-2.269 | 0.498 |
Table 5
Detection Rate of High-risk Adenoma Based on the Follow-up Period from the Last Colonoscopy and Previous Colonoscopy Findings
| Patients | Number of high-risk adenoma cases / Number of cases with previous CFS < 5 year | Number of high-risk adenoma cases / Number of cases with previous CFS ≥ 5 year | p-valuea |
|---|---|---|---|
| Total (n=13,396) | 727/7,197 (10.1) | 300/2,438 (12.3) | 0.002b |
| Polyp surveillance group (n=2,274) | 248/1,995 (12.4) | 54/279 (19.4) | 0.015b |
| Normal index CFS group (n=5,156) | 298/3,316 (9.0) | 205/1,840 (11.1) | 0.012b |
Table 6
Logistic Regression Analysis of the Risk Factors of High-risk Adenoma: Previous Normal Colonoscopy Conducted within 5 Years
| Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|
|
|
|
||||
| OR | p-value | OR | 95% CI | p-value | |
| Sex | |||||
| Female | 1 (referent) | ||||
| Male | 1.849 | 0.000a | 1.968 | 1.536-2.523 | 0.000a |
| Age numerical | 1.039 | 0.000a | 1.04 | 1.028-1.052 | 0.000a |
| DM | |||||
| (-) | 1 (referent) | ||||
| (+) | 1.236 | 0.248 | 0.98 | 0.676-1.422 | 0.916 |
| CRC FHx | |||||
| (-) | 1 (referent) | ||||
| (+) | 1.159 | 0.68 | 1.386 | 0.677-2.839 | 0.372 |
| BMI | |||||
| <25 | 1 (referent) | ||||
| ≥25 | 1.106 | 0.429 | 1.076 | 0.833-1.389 | 0.575 |
| FOBT | |||||
| (-) | 1 (referent) | ||||
| (+) | 1.932 | 0.003a | 1.929 | 1.233-3.018 | 0.004a |
| Abdominal pain | |||||
| (-) | 1 (referent) | ||||
| (+) | 0.934 | 0.681 | 1.108 | 0.792-1.551 | 0.549 |
| Bloody stool | |||||
| (-) | 1 (referent) | ||||
| (+) | 0.883 | 0.616 | 1.126 | 0.682-1.859 | 0.644 |
| Constipation | |||||
| (-) | 1 (referent) | ||||
| (+) | 1.138 | 0.465 | 1.244 | 0.870-1.778 | 0.231 |
| Diarrhea | |||||
| (-) | 1 (referent) | ||||
| (+) | 0.973 | 0.874 | 0.946 | 0.672-1.332 | 0.75 |
| Stool form changes | |||||
| (-) | 1 (referent) | ||||
| (+) | 1.584 | 0.007a | 1.552 | 1.098-2.195 | 0.013a |
| Anemia | |||||
| (-) | 1 (referent) | ||||
| (+) | 1.217 | 0.749 | 1.397 | 0.404-4.836 | 0.598 |
| Weight loss | |||||
| (-) | 1 (referent) | ||||
| (+) | 1.384 | 0.395 | 1.376 | 0.639-2.965 | 0.415 |
| Nonspecific symptom (gas, indigestion) | |||||
| (-) | 1 (referent) | ||||
| (+) | 1.351 | 0.014a | 1.445 | 1.121-1.863 | 0.004a |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download