Abstract
Background/Aims
This multicenter study reviewed the clinical features and prognosis according to the primary site of involvement and the treatment modality in patients with B-cell primary intestinal lymphoma (PIL).
Results
The median age was 59 years, and the male to female ratio was 1.86:1. Diffuse large B-cell lymphoma (66/100, 66.0%) was the most common histological subtype. The estimated 5-year survival rate (5-YSR) was 48.5%. The 5-YSR was similar regardless of the type of primary treatment (chemotherapy alone vs. surgery/chemotherapy, 50.7 vs. 45.3%, p=0.582). A comparison of the survival according to the primary site of involvement revealed a 5-YSR of 32.5% (p=0.027), 64.3% (reference), 46.5% (p=0.113), and 49.8% (p=0.024) for the small intestine, ileocecal region, large intestine, and multiple sites, respectively. Multivariate analysis, however, revealed a low hemoglobin level, advanced Ann Arbor stage, and aggressive histological type to be independent prognostic factors for shorter survival but not ileocecal region involvement.
The gastrointestinal (GI) tract is the most common extranodal site of involvement in non-Hodgkin’s lymphoma (NHL).1,2 Primary intestinal lymphomas (PILs), which include lymphomas of the small intestine and large bowel, were the second most common sites of involvement following the stomach and accounted for 20-30% of all GI lymphomas with an increasing incidence.3 The clinical features, pathology findings, treatments, and prognosis of PILs differ from gastric lymphomas.4-8 While substantial progress has been made in the diagnosis and treatment of gastric lymphoma,9,10 a limited number of studies of PIL have been reported because PIL is a rare and more heterogeneous disease with regard to the anatomical sites and histological subtypes.11-14 A prospective, multicenter study reported the distinct clinical features of 91 patients with primary intestinal B-cell lymphoma according to the histological subtype; one-third of patients were diagnosed surgically. The survival outcome was described, but the impact of the treatment strategy on the prognosis was not reported.11 A large, retrospective study of 561 patients with PIL reported that surgery plus chemotherapy was associated with better survival than chemotherapy alone in B-cell intestinal lymphoma. Multivariate analysis, however, showed that a surgical resection was not an independent prognostic factor for the overall survival (OS).12 Recently, a population-based study of 1,602 patients with primary intestinal diffuse large B-cell lymphoma (DLBCL) reported that regardless of the type of surgery, combined treatment with surgery and chemotherapy improved the survival outcomes compared to chemotherapy alone. On the other hand, there was a lack of information on ECOG PS, LDH, International Prognostic Index (IPI), and disease status (at diagnosis or relapsed), making it difficult to assess the balance of the clinical characteristics between patients having different treatment strategies.15
Therefore, this study examined the clinical features and survival outcomes according to the anatomical site in patients with PIL limited to the B-cell lineage (B-cell PIL) in this multi-center retrospective study. Furthermore, the impacts of surgical resection, the purpose of surgery, and conventional prognostic factors on survival were also assessed.
This paper reviewed the data for 125 consecutive patients with PIL collected from six Korean institutions between January 2001 and March 2014. The inclusion criteria were as follows: 1) histologically confirmed diagnosis of NHL based on the World Health Organization classification; 2) documentation of PIL without extranodal involvement other than the intestine by imaging modalities, such as CT, MRI, or PET. Among the 125 patients reviewed, 21 patients with T-cell NHL and four patients not treated with chemotherapy were excluded. Finally, 100 patients were included and analyzed. The Institutional Review Board (IRB) of Gyeongsang National University Changwon Hospital (IRB No. 2017-08-009-002) at each participating institution approved the study protocol. The ethics committee waived the requirement for informed consent of medical records used in the present study.
The data collected included the patients’ demographics and the clinical features at diagnosis, including the Eastern Cooperative Oncology Group performance status (ECOG PS), presence of B symptoms and intestinal complications (bleeding, obstruction, or perforation), laboratory findings (LDH and hemoglobin levels), tumor location, and histological subtype. The tumor was staged using the Ann Arbor and Lugano staging systems. Between the two staging systems, the Ann Arbor stage, together with age, ECOG PS, LDH, and the number of extranodal involvement sites, were used to determine the IPI. The type of primary treatment and its impact on survival were also reviewed.
The definition of B-cell PIL was B-cell NHL localized to the intestinal tract from the duodenum to the rectum, with or without nodal disease. The tumor locations were determined using image modalities or surgical pathology findings if a surgical resection was performed. The small intestine was defined as the duodenum, jejunum, and ileum. The large intestine included the segment of the bowel from the ascending colon to the rectum. The ileocecal (IC) region was defined as the area between the distal ileum and cecum.12 Multiple site involvement was defined as a case in which there was combined involvement of the small intestine and large bowels B-cell lymphoma (MZL) and follicular lymphoma (FL). DLBCL, and IC region. An indolent histology included marginal zone mantle cell lymphoma (MCL), and Burkitt lymphoma (BL) were defined as aggressive histologies.
All statistical analyses were performed using SPSS version
21.0 software (SPSS, Chicago, IL, USA). The chi-square and Fisher exact tests were performed for the categorical data to identify the possible differences between the groups. The median follow-up time was calculated using the reverse Kaplan-Meier method.16 OS was calculated from the date of diagnosis to the date of the last follow-up or death from any cause. Survival was estimated using the Kaplan-Meier curves. The Cox proportional hazard regression model with the enter selection method was used in multivariate analysis to identify the prognostic factors. All variables with p<0.10 were included in multivariate analysis. Two-tailed p-values less than 0.05 were considered significant.
Table 1 lists the patients’ characteristics. In general, the median age was 59 years (range 9-82 years), and the male to female ratio was 1.86:1. Most patients had a good performance status (ECOG grade 0 or 1, 81.0%) and elevated serum LDH level (78.0%). Approximately half of the patients (52.0%) were Ann Arbor stage I-II. The proportion of stage I-II was higher (77.0%) in the Lugano staging system. More patients had low to low-intermediate IPI scores (63.0%). B symptoms and lymphoma-related complications were observed in 14 and 24 patients, respectively. Among the lymphoma-related complications observed, bleeding was a predominant complication (14/24, 58.3%), followed by obstruction (9/24, 37.5%) and perforation (1/24, 4.2%). DLBCL (66.0%) was the most common histological subtype. Most patients received chemotherapy alone as the frontline treatment (74.0%), and the others were treated with combined surgery and chemotherapy (26.0%). Fourteen of the 26 patients (53.8%) who were treated with combined surgery and chemotherapy and 14 of the 76 (18.4%) who did not have lymphoma-related complications received surgery for diagnostic or therapeutic purposes without lymphoma-related complications. Twelve of the 24 (50.0%) who had lymphoma-related complications received emergent or urgent surgery. Among the 94 patients for whom the information for specific chemotherapy regimen was available, 76 patients (80.9%) were treated with a rituximab-containing regimen.
The patients’ characteristics were compared according to the primary site of the lymphoma (Table 1). The large intestine was involved more often in B-cell lymphoma (28.0%) than the small intestine (18.0%) and IC region (18.0%). Multiple site involvement was observed in 36 patients (36.0%). There were no significant differences in the distribution of clinical factors between the groups. On the other hand, multiple site involvement was associated with a higher proportion of MCL (12/36, 33.3%), and BL was observed more frequently in the IC region (3/18, 16.7%). A low hemoglobin level (hemoglobin ≤10 g/dL) and advanced Ann Arbor stage (stage III-IV) were observed less frequently in the IC region, but the differences were not significant.
With a median follow-up of 44.0 months (range 1-202 months), 44 deaths were noted. In 100 patients, the median OS was 50.0 months (95% CI 32.8-67.2 months), and the estimated 5-year survival rate (5-YSR) was 48.5% (Fig. 1). When the OS was compared according to the primary site of the lymphoma, patients with IC region involvement had a longer OS than those with involvement of other sites (Fig. 2A). The estimated 5-YSR was 32.5% (p=0.027), 64.3% (reference), 46.5% (p=0.113), and 49.8% (p=0.024) in the small intestine, IC region, large intestine, and multiple site involvement groups, respectively. On the other hand, the estimated 5-YSR were similar regardless of the type of primary treatment (chemotherapy alone vs. surgery/chemotherapy, 50.7% vs. 45.3%, p=0.582) (Fig. 2B). There were also no differences in the 5-YSR regardless of the purpose of surgery (emergent/urgent vs. diagnostic/therapeutic, 45.5% vs. 44.0%, p=0.526) and treatment strategy in the absence of lymphoma-related complication (chemotherapy alone vs. surgery/chemotherapy, 56.8% vs. 44.0%, p=0.242).
Univariate analyses revealed ECOG PS, LDH and hemoglobin level, Ann Arbor stage, histological type, and primary site of lymphoma to be potential prognostic factors for OS. Multivariate analysis, however, revealed hemoglobin level ≤10 g/dL, Ann Arbor stage III-IV, and aggressive histological type to be independent prognostic factors for a poorer OS (Table 2).
This study investigated the clinical features and prognosis of 100 patients with B-cell PIL. A similar distribution of clinical characteristics was observed in previous studies and the present work. A male predominance was noted in this population, as reported elsewhere.12,17 DLBCL was the most common histological subtype (66.0%). This incidence is similar to that (46.0-86.0%) reported in previous studies.12,17-20 MCL was related mostly to multiple intestinal involvement, as reported in the literature.21,22 Furthermore, clinical features associated with advanced disease, such as poor PS, advanced Lugano stage, and high-intermediate to high IPI, were observed less frequently in patients with B-cell PIL in previous studies and the present study.12,17,20 By contrast, there was a difference in survival outcome between previous studies and the present study. The 5-YSR (48.5%) in the present study was lower than that (55.0-77.0%) reported in previous studies.11,12,18,19 This difference may be due partly to a relatively higher proportion of advanced tumor stage and unfavorable histological subtypes, such as MCL in the present study than in previous studies.
As reported elsewhere,11,12,18 advanced tumor stage and aggressive histological subtype were associated with a poor prognosis in the present study. In unadjusted analysis, single involvement of the IC region was also associated with a favorable prognosis compared to other site involvement. This finding is consistent with previous studies showing favorable survival outcomes in patients with involvement of only the IC region.5,12,23 Kim et al.12 explained the superior survival outcomes in patients with IC region involvement by the rarity of T-cell lymphoma and a higher proportion of surgical intervention among these patients. In the present study, however, patients with T-cell lymphoma were excluded, and surgical intervention did not improve the survival outcome. Furthermore, multivariate analysis showed no significant difference in OS according to the primary site of involvement. Therefore, the primary site of involvement itself may not be an independent prognostic factor for survival in patients with B-cell PIL. Instead, a lower proportion of advanced Ann Arbor stage and a low hemoglobin level observed in patients with IC region involvement may have a favorable influence on survival according to univariate analysis.
A low hemoglobin level was an independent prognostic factor for a poor prognosis in this study. Although anemia is not a component of IPI, its prognostic value has been evaluated in patients with NHL. Owing to the multifactorial pathogenesis of anemia in patients with lymphoma, the reason why anemia is associated with a poor prognosis remains to be determined.24 Elevated interleukin (IL)-6, which plays a major role in the development of anemia, is an independent prognostic factor in patients with DLBCL.24-26 Other inflammatory cytokines produced by lymphoma cells, such as IL-1, IL-10, and tumor necrosis factor-α, reduce erythropoietin production and inhibit the response of erythroid progenitors to erythropoietin.27 A recent study reported that anemia was an independent predictor of impaired OS in patients with DLBCL.24 Another study showed that hemoglobin <10 g/dL was an IPI-independent prognostic factor in patients with DLBCL treated with immunochemotherapy.28 These studies support the present findings that low hemoglobin levels may negatively influence the outcome of patients with B-cell lymphoma. Nevertheless, whether a low hemoglobin level is a poor prognostic factor in PIL needs to be determined.
The optimal treatment strategy in PIL has not been established. Although the treatment modalities for PIL are controversial, surgical resection has been historically accepted as the primary treatment strategy. Several previous studies reported that surgery is associated with a favorable outcome.14,29-31 Some studies reported a high response rate and 5-YSR of more than 70.0% with chemotherapy alone, and no difference in survival following chemotherapy vs. surgery combined with chemotherapy in patients with PIL.32-34 In a retrospective study of 345 patients with primary intestinal DLBCL, combined treatment with surgery and chemotherapy did not improve the survival compared to chemotherapy alone in those with disseminated disease.14 The present study revealed similar survival rates between patients treated with chemotherapy alone and those treated with combined surgery and chemotherapy. Given the heterogeneity of the histological subtype and the small sample size in the present study, while the role of surgery cannot be denied in localized PIL, surgery might be reserved for patients with intestinal complications in non-localized intestinal PIL.
This study had some limitations. First, the study was a retrospective analysis. Therefore, not all required data was available, including progression-free survival, the cell-of-origin, treatment-related adverse events, and endoscopic or surgical gross findings of tumor. Second, the present study included a small number of patients with heterogeneous histologies. In particular, only 26 patients received the combined treatment with surgery and chemotherapy. These limitations made it difficult to analyze the survival according to the histological subtype and treatment modality.
In conclusion, the clinical features and outcomes of patients with B-cell PIL were examined. While an advanced Ann Arbor stage, low hemoglobin level, and aggressive histological type were independent poor prognostic factors for the OS, the primary site of involvement and the treatment modality did not affect the OS. Given the heterogeneity of patients in this study, a large and prospective study of patients with a homogeneous histological subtype will be needed to validate these findings.
REFERENCES
1. d'Amore F, Brincker H, Grønbaek K, et al. 1994; Non-Hodgkin's lymphoma of the gastrointestinal tract: a population-based analysis of incidence, geographic distribution, clinicopathologic presentation features, and prognosis. Danish Lymphoma Study Group. J Clin Oncol. 12:1673–1684. DOI: 10.1200/JCO.1994.12.8.1673. PMID: 8040680.
2. Ko YH, Kim CW, Park CS, et al. 1998; REAL classification of malignant lymphomas in the Republic of Korea: incidence of recently recognized entities and changes in clinicopathologic features. Hematolymphoreticular Study Group of the Korean Society of Pathologists. Revised European-American lymphoma. Cancer. 83:806–812. DOI: 10.1002/(SICI)1097-0142(19980815)83:4<806::AID-CNCR26>3.0.CO;2-V. PMID: 9708949.
3. Lepage C, Bouvier AM, Manfredi S, Dancourt V, Faivre J. 2006; Incidence and management of primary malignant small bowel cancers: a well-defined French population study. Am J Gastroenterol. 101:2826–2832. DOI: 10.1111/j.1572-0241.2006.00854.x. PMID: 17026561.

4. Dragosics B, Bauer P, Radaszkiewicz T. 1985; Primary gastrointestinal non-Hodgkin's lymphomas. A retrospective clinicopathologic study of 150 cases. Cancer. 55:1060–1073. DOI: 10.1002/1097-0142(19850301)55:5<1060::AID-CNCR2820550523>3.0.CO;2-8.

5. Koch P, del Valle F, Berdel WE, et al. 2001; Primary gastrointestinal non-Hodgkin's lymphoma: I. Anatomic and histologic distribution, clinical features, and survival data of 371 patients registered in the German Multicenter Study GIT NHL 01/92. J Clin Oncol. 19:3861–3873. DOI: 10.1200/JCO.2001.19.18.3861. PMID: 11559724.

6. Ruskoné-Fourmestraux A, Aegerter P, Delmer A, Brousse N, Galian A, Rambaud JC. 1993; Primary digestive tract lymphoma: a prospective multicentric study of 91 patients. Groupe d'Etude des Lymphomes Digestifs. Gastroenterology. 105:1662–1671. DOI: 10.1016/0016-5085(93)91061-L.
7. Fischbach W, Dragosics B, Kolve-Goebeler ME, et al. 2000; Primary gastric B-cell lymphoma: results of a prospective multicenter study. The German-Austrian Gastrointestinal Lymphoma Study Group. Gastroenterology. 119:1191–1202. DOI: 10.1053/gast.2000.19579. PMID: 11054376.
8. Nakamura S, Matsumoto T, Iida M, Yao T, Tsuneyoshi M. 2003; Primary gastrointestinal lymphoma in Japan: a clinicopathologic analysis of 455 patients with special reference to its time trends. Cancer. 97:2462–2473. DOI: 10.1002/cncr.11415. PMID: 12733145.
9. Ruskoné-Fourmestraux A, Fischbach W, Aleman BM, et al. 2011; EGILS consensus report. Gastric extranodal marginal zone B-cell lymphoma of MALT. Gut. 60:747–758. DOI: 10.1136/gut.2010.224949. PMID: 21317175.

10. Zucca E. ESMO Guidelines Working Group. 2007; Gastric marginal zone lymphoma of mucosa-associated lymphoid tissue type: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 18(Suppl 2):ii59–ii60. DOI: 10.1093/annonc/mdm039. PMID: 17491050.

11. Matysiak-Budnik T, Jamet P, Fabiani B, et al. 2013; Primary intestinal B-cell lymphoma: a prospective multicentre clinical study of 91 cases. Dig Liver Dis. 45:947–952. DOI: 10.1016/j.dld.2013.05.008. PMID: 23816692.

12. Kim SJ, Choi CW, Mun YC, et al. 2011; Multicenter retrospective analysis of 581 patients with primary intestinal non-hodgkin lymphoma from the Consortium for Improving Survival of Lymphoma (CISL). BMC Cancer. 11:321. DOI: 10.1186/1471-2407-11-321. PMID: 21798075. PMCID: PMC3160411.

13. Gurney KA, Cartwright RA, Gilman EA. 1999; Descriptive epidemiology of gastrointestinal non-Hodgkin's lymphoma in a population-based registry. Br J Cancer. 79:1929–1934. DOI: 10.1038/sj.bjc.6690307. PMID: 10206316. PMCID: PMC2362786.

14. Kim SJ, Kang HJ, Kim JS, et al. 2011; Comparison of treatment strategies for patients with intestinal diffuse large B-cell lymphoma: surgical resection followed by chemotherapy versus chemotherapy alone. Blood. 117:1958–1965. DOI: 10.1182/blood-2010-06-288480. PMID: 21148334.

15. Wang M, Ma S, Shi W, Zhang Y, Luo S, Hu Y. 2021; Surgery shows survival benefit in patients with primary intestinal diffuse large B-cell lymphoma: a population-based study. Cancer Med. 10:3474–3485. DOI: 10.1002/cam4.3882. PMID: 33931950. PMCID: PMC8124121.

16. Schemper M, Smith TL. 1996; A note on quantifying follow-up in studies of failure time. Control Clin Trials. 17:343–346. DOI: 10.1016/0197-2456(96)00075-X. PMID: 8889347.

17. Li B, Shi YK, He XH, et al. 2008; Primary non-Hodgkin lymphomas in the small and large intestine: clinicopathological characteristics and management of 40 patients. Int J Hematol. 87:375–381. DOI: 10.1007/s12185-008-0068-5. PMID: 18409078.

18. Nakamura S, Matsumoto T, Takeshita M, et al. 2000; A clinicopathologic study of primary small intestine lymphoma: prognostic significance of mucosa-associated lymphoid tissue-derived lymphoma. Cancer. 88:286–294. DOI: 10.1002/(SICI)1097-0142(20000115)88:2<286::AID-CNCR7>3.0.CO;2-Z. PMID: 10640959.
19. Wang GB, Xu GL, Luo GY, et al. 2011; Primary intestinal non-Hodgkin's lymphoma: a clinicopathologic analysis of 81 patients. World J Gastroenterol. 17:4625–4631. DOI: 10.3748/wjg.v17.i41.4625. PMID: 22147970. PMCID: PMC3226984.

20. Daum S, Ullrich R, Heise W, et al. 2003; Intestinal non-Hodgkin's lymphoma: a multicenter prospective clinical study from the German Study Group on Intestinal non-Hodgkin's Lymphoma. J Clin Oncol. 21:2740–2746. DOI: 10.1200/JCO.2003.06.026. PMID: 12860953.

21. Kodama T, Ohshima K, Nomura K, et al. 2005; Lymphomatous polyposis of the gastrointestinal tract, including mantle cell lymphoma, follicular lymphoma and mucosa-associated lymphoid tissue lymphoma. Histopathology. 47:467–478. DOI: 10.1111/j.1365-2559.2005.02225.x. PMID: 16241994.

22. Salar A, Juanpere N, Bellosillo B, et al. 2006; Gastrointestinal involvement in mantle cell lymphoma: a prospective clinic, endoscopic, and pathologic study. Am J Surg Pathol. 30:1274–1280. DOI: 10.1097/01.pas.0000208899.15859.cb. PMID: 17001159.

23. Lee J, Kim WS, Kim K, et al. 2004; Intestinal lymphoma: exploration of the prognostic factors and the optimal treatment. Leuk Lymphoma. 45:339–344. DOI: 10.1080/10428190310001593111. PMID: 15101721.

24. Troppan KT, Melchardt T, Deutsch A, et al. 2015; The significance of pretreatment anemia in the era of R-IPI and NCCN-IPI prognostic risk assessment tools: a dual-center study in diffuse large B-cell lymphoma patients. Eur J Haematol. 95:538–544. DOI: 10.1111/ejh.12529. PMID: 25677782.

25. Tisi MC, Bozzoli V, Giachelia M, et al. 2014; Anemia in diffuse large B-cell non-Hodgkin lymphoma: the role of interleukin-6, hepcidin and erythropoietin. Leuk Lymphoma. 55:270–275. DOI: 10.3109/10428194.2013.802314. PMID: 23647063.

26. Giachelia M, Voso MT, Tisi MC, et al. 2012; Interleukin-6 plasma levels are modulated by a polymorphism in the NF-κB1 gene and are associated with outcome following rituximab-combined chemotherapy in diffuse large B-cell non-Hodgkin lymphoma. Leuk Lymphoma. 53:411–416. DOI: 10.3109/10428194.2011.621566. PMID: 21902578.
27. Suzuki K, Terui Y, Nishimura N, et al. 2013; Prognostic value of C-reactive protein, lactase dehydrogenase and anemia in recurrent or refractory aggressive lymphoma. Jpn J Clin Oncol. 43:37–44. DOI: 10.1093/jjco/hys194. PMID: 23166385.

28. Hong J, Woo HS, Kim H, et al. 2014; Anemia as a useful biomarker in patients with diffuse large B-cell lymphoma treated with R-CHOP immunochemotherapy. Cancer Sci. 105:1569–1575. DOI: 10.1111/cas.12544. PMID: 25263825. PMCID: PMC4317957.
29. Gobbi PG, Ghirardelli ML, Cavalli C, et al. 2000; The role of surgery in the treatment of gastrointestinal lymphomas other than low-grade MALT lymphomas. Haematologica. 85:372–380. PMID: 10756362.
30. Ibrahim EM, Ezzat AA, El-Weshi AN, et al. 2001; Primary intestinal diffuse large B-cell non-Hodgkin's lymphoma: clinical features, management, and prognosis of 66 patients. Ann Oncol. 12:53–58. DOI: 10.1023/A:1008389001990. PMID: 11249049.

31. Zinzani PL, Magagnoli M, Pagliani G, et al. 1997; Primary intestinal lymphoma: clinical and therapeutic features of 32 patients. Haematologica. 82:305–308. PMID: 9234576.
32. Kobayashi H, Nagai T, Omine K, et al. 2013; Clinical outcome of non-surgical treatment for primary small intestinal lymphoma diagnosed with double-balloon endoscopy. Leuk Lymphoma. 54:731–736. DOI: 10.3109/10428194.2012.725850. PMID: 22946663.

33. Shawky H, Tawfik H. 2008; Primary gastrointestinal non-Hodgkin's lymphoma: a retrospective study with emphasis on prognostic factors and treatment outcome. J Egypt Natl Canc Inst. 20:330–341.
34. Lee HS, Park LC, Lee EM, et al. 2014; Comparison of therapeutic outcomes between surgical resection followed by R-CHOP and R-CHOP alone for localized primary intestinal diffuse large B-cell lymphoma. Am J Clin Oncol. 37:182–187. DOI: 10.1097/COC.0b013e318271b125. PMID: 23211226.

Fig. 1
Kaplan-Meier estimates of the overall survival in all patients with primary intestinal B-cell lymphoma.
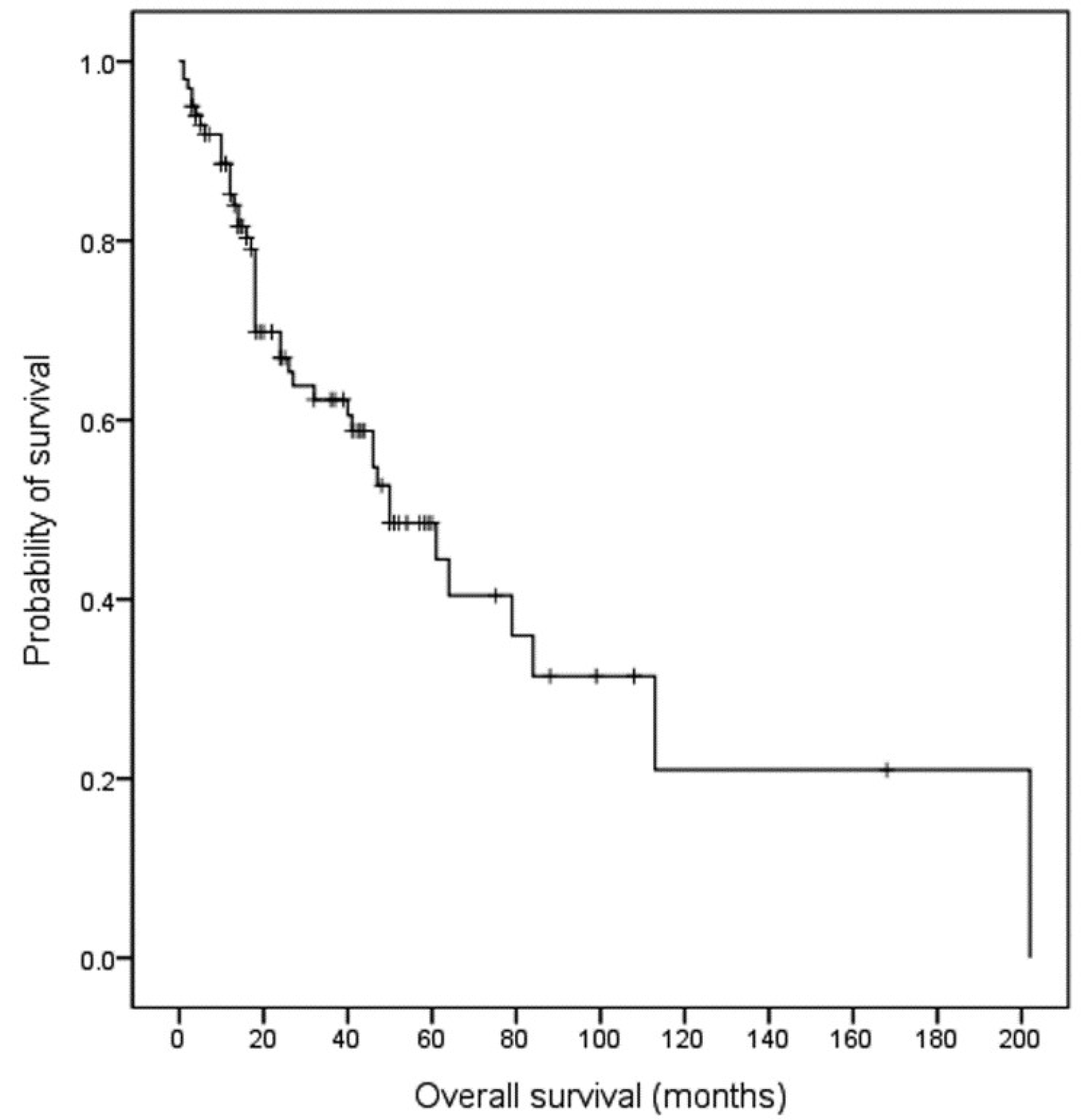
Fig. 2
Kaplan-Meier estimates of the overall survival according to (A) the primary site of involvement and (B) the type of primary treatment. ns, not significant.
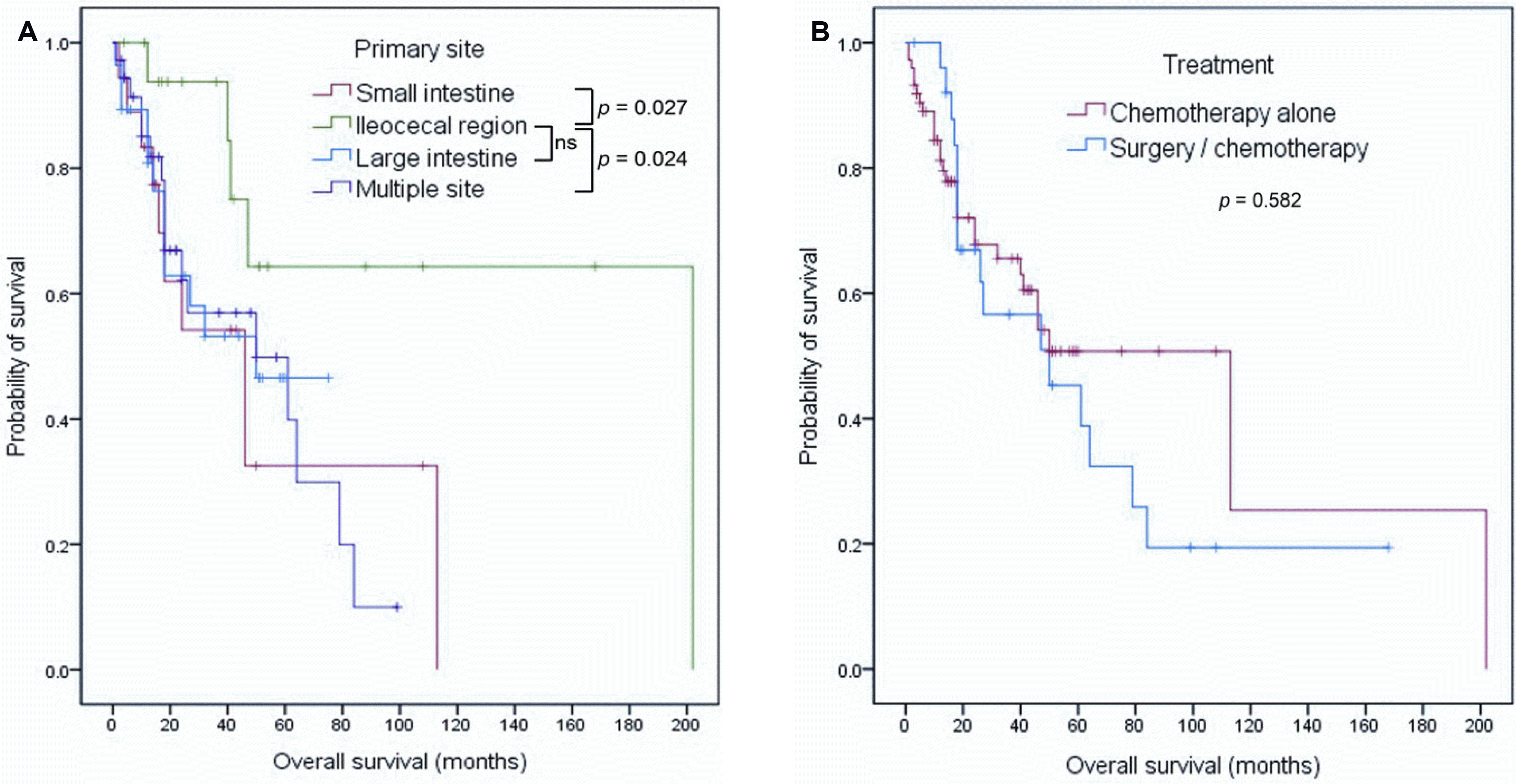
Table 1
Patients’ Characteristics
| Variable | Total (n=100) | Small intestine (n=18) | IC region (n=18) | Large intestine (n=28) | Multiple site (n=36) | p-value |
|---|---|---|---|---|---|---|
| Sex | 0.397 | |||||
| Male | 65 | 13 (72.2) | 11 (61.1) | 15 (53.6) | 26 (72.2) | |
| Female | 35 | 5 (27.8) | 7 (38.9) | 13 (46.4) | 10 (27.8) | |
| Age (years) | 0.887 | |||||
| <60 | 51 | 9 (50.0) | 9 (50.0) | 16 (57.1) | 17 (47.2) | |
| ≥60 | 49 | 9 (50.0) | 9 (50.0) | 12 (42.9) | 19 (52.8) | |
| ECOG PS | 0.143a | |||||
| <2 | 81 | 12 (66.7) | 14 (77.8) | 26 (92.9) | 29 (80.6) | |
| ≥2 | 19 | 6 (33.3) | 4 (22.2) | 2 (7.1) | 7 (19.4) | |
| LDH | 0.252a | |||||
| Normal | 22 | 6 (33.3) | 2 (11.1) | 4 (14.3) | 10 (27.8) | |
| Increased | 78 | 12 (66.7) | 16 (88.9) | 24 (85.7) | 26 (72.2) | |
| Hemoglobin | 0.547a | |||||
| ≤10 g/dL | 26 | 6 (33.3) | 3 (16.7) | 9 (32.1) | 8 (22.2) | |
| >10 g/dL | 74 | 12 (66.7) | 15 (83.3) | 19 (67.9) | 28 (77.8) | |
| Ann Arbor stage | 0.379 | |||||
| I-II | 52 | 8 (44.4) | 12 (66.7) | 16 (57.1) | 16 (44.4) | |
| III-IV | 48 | 10 (55.6) | 6 (33.3) | 12 (42.9) | 20 (55.6) | |
| Lugano stage | 0.973a | |||||
| I-II | 77 | 13 (72.2) | 14 (77.8) | 22 (78.6) | 28 (77.8) | |
| IV | 23 | 5 (27.8) | 4 (22.2) | 6 (21.4) | 8 (22.2) | |
| IPI | 0.324 | |||||
| L/LI | 63 | 9 (50.0) | 12 (66.7) | 21 (75.0) | 21 (58.3) | |
| HI/H | 37 | 9 (50.0) | 6 (33.3) | 7 (25.0) | 15 (41.7) | |
| B symptoms | 0.077a | |||||
| Absent | 86 | 12 (66.7) | 17 (94.4) | 26 (92.9) | 31 (86.1) | |
| Present | 14 | 6 (33.3) | 1 (5.6) | 2 (7.1) | 5 (13.9) | |
| Complicationb | 0.211a | |||||
| Absent | 76 | 12 (66.7) | 11 (61.1) | 23 (82.1) | 30 (83.3) | |
| Present | 24 | 6 (33.3) | 7 (38.9) | 5 (17.9) | 6 (16.7) | |
| Histology | 0.013a | |||||
| DLBCL | 66 | 14 (77.8) | 12 (66.7) | 22 (78.6) | 18 (50.0) | |
| MCL | 15 | 2 (11.1) | 0 (0.0) | 1 (3.6) | 12 (33.3) | |
| MZL | 12 | 2 (11.1) | 3 (16.7) | 3 (10.7) | 4 (11.1) | |
| BL | 6 | 0 (0.0) | 3 (16.7) | 2 (7.1) | 1 (2.8) | |
| FL | 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.8) | |
| Frontline treatment | 0.137a | |||||
| Chemotherapy alone | 74 | 16 (88.9) | 11 (61.1) | 23 (82.1) | 24 (66.7) | |
| Surgery/chemotherapy | 26 | 2 (11.1) | 7 (38.9) | 5 (17.9) | 12 (33.3) |
IC, ileocolic; ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; IPI, International Prognostic Index; L/LI, low/low-intermediate; HI/H, high-intermediate/high; DLBCL, diffuse large B-cell lymphoma; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; BL, Burkitt lymphoma; FL, follicular lymphoma.
Table 2
Univariate and Multivariate Analysis for the Overall Survival
| Variable | Univariatea | Multivariate | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Sex (male vs. female) | 1.288 | 0.680-2.441 | 0.438 | |||
| Age (≥60 years vs. <60 years) | 1.218 | 0.663-2.237 | 0.525 | |||
| ECOG PS (≥2 vs. <2) | 2.242 | 1.103-4.557 | 0.026 | 1.769 | 0.805-3.887 | 0.155 |
| LDH (elevated vs. normal) | 0.570 | 0.307-1.055 | 0.073 | 0.670 | 0.347-1.291 | 0.231 |
| Hemoglobin (≤10 g/dL vs. >10 g/dL) | 2.547 | 1.352-4.798 | 0.004 | 2.119 | 1.044-4.303 | 0.038 |
| Ann Arbor stage (III-IV vs. I-II) | 2.037 | 1.109-3.741 | 0.022 | 2.092 | 1.102-3.971 | 0.024 |
| Lugano stage (IV vs. I-II) | 0.798 | 0.365-1.743 | 0.571 | |||
| B symptoms (present vs. absent) | 1.248 | 0.551-2.828 | 0.595 | |||
| Complication (present vs. absent) | 1.095 | 0.561-2.138 | 0.790 | |||
| Histology (aggressive vs. indolent)b | 3.611 | 0.868-15.019 | 0.077 | 4.323 | 1.029-18.156 | 0.046 |
| Treatment (chemotherapy alone vs. surgery/chemotherapy) | 0.841 | 0.449-1.573 | 0.587 | |||
| Primary site (IC region vs. others) | 0.295 | 0.104-0.842 | 0.022 | 0.408 | 0.138-1.206 | 0.105 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download