This article has been
cited by other articles in ScienceCentral.
Abstract
Objective
This study retrospectively reviewed the process of classifying antineutrophil cytoplasmic antibody (ANCA)-negative granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) in a cohort of patients with ANCA-associated vasculitis (AAV), and investigated the association between recurrent idiopathic cutaneous leukocytoclastic angiitis and ANCA-negative MPA
Methods
The medical records of 242 patients with AAV were retrospectively reviewed Of 49 patients with ANCA-negative AAV, 24 patients with ANCA-negative eosinophilic GPA (EGPA) were excluded, because ANCA positivity or negativity is not critical in classifying EGPA Ultimately, 25 patients with ANCA-negative GPA and MPA were analysed in this study The classification of GPA and MPA were based on the 2007 European Medicines Agency algorithm for AAV
Results
The median age of patients with ANCA-negative GPA and MPA was 540 years and 24% were male Of the 25 patients without ANCA, 8 patients were classified as GPA and 17 as MPA Eight patients with ANCA-negative GPA were easily confirmed as definitive GPA Fourteen of the 17 patients ANCA-negative MPA were classified as MPA based on histological features suggestive of AAV without granuloma formation and the absence of surrogate markers for GPA Meanwhile, three of the patients that were ANCA-negative exhibited only recurrent idiopathic cutaneous leukocytoclastic angiitis without other major organs affected and thus were classified as possible MPA Within one year, they were classified as definitive MPA based on ANCA positivity and/or renal histology
Conclusion
Recurrent idiopathic cutaneous leukocytoclastic angiitis may be associated with ANCA-negative MPA in patients who exhibit cutaneous necrotising vasculitis
Keywords: Idiopathic, Leukocytoclastic, Vasculitis, Microscopic polyangiitis, Anti-neutrophil cytoplasmic antibody
INTRODUCTION
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is classified as a type of necrotising vasculitis with few or no immune deposits in small-size vessels. AAV primarily involves the capillaries, pre-capillary arterioles and post-capillary venules and occasionally affects arteries [
1]. Based on clinical, laboratory, radiological and histological features, AAV is classified into three subtypes: microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA) and eosinophilic GPA (EGPA) [
1,
2].
As the name of AAV represents, ANCA positivity is a cornerstone in the diagnosis of AAV. In cases with ANCA positivity, it is not difficult to suspect AAV and subsequently perform the requisite tests for AAV diagnosis. However, in cases with ANCA negativity, it is not easy to initially suspect AAV [
2]. In addition, when histological features are insufficient to definitively classify AAV, ANCA negativity discourages physicians from taking a further step forward AAV diagnosis. Nevertheless, because the number of suspected cases of ANCA-negative AAV is increasing, it is important to be aware of the possibility of ANCA-negative AAV and make attempts to detect ANCA using novel alternative methods [
3]. Additionally, regarding the classification of EGPA, ANCA results are not essential [
4]. In contrast, when classifying MPA and GPA, ANCA results may be crucial according to histological features suggesting MPA or GPA and surrogate markers for GPA [
2]. Furthermore, while the clinical value of ANCA in patient prognosis still remains controversial, it has been reported that ANCA could be a predictor of disease relapse in patients with AAV with renal involvement [
5].
In real-world clinical practice, we may encounter patients with cutaneous leukocytoclastic angiitis with histological features of necrotising vasculitis involving pre-capillary arterioles, capillaries and post-capillary venules without evidence of immune deposits [
1,
6]. In particular, a dilemma emerges if the cause of cutaneous vasculitis is unclear, and the patient exhibits persistent recurrence, and no major organ involvement other than the skin. We may follow up these patients and perform tests to discriminate AAV and polyarteritis nodosa, or alternatively, consider it to be a specific disease confined to the skin and refer them to a dermatologist [
7,
8]. In the present study, we retrospectively reviewed the process of classifying ANCA-negative GPA or MPA in a cohort of AAV patients and investigated the association between recurrent idiopathic cutaneous leukocytoclastic angiitis and ANCA-negative MPA.
MATERIALS AND METHODS
Subjects
The medical records of 242 patients with AAV were retrospectively reviewed. All patients fulfilled the 1990 American College of Rheumatology criteria for the classification of Churg-Strauss syndrome, the 2007 European Medicines Agency algorithm for AAV and polyarteritis nodosa and the 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides [
1,
2,
4]. Patients who were followed up for less than 3 months and had concomitant serious medical conditions such as malignancies, serious infections, autoimmune systemic vasculitis other than AAV, or irreversible major organ damage caused by AAV at diagnosis, were excluded from this study [
1,
9,
10]. Of the 242 AAV patients, 193 were excluded due to ANCA positivity and among the 49 patients with ANCA-negative AAV, 24 patients with ANCA-negative EGPA were excluded, because ANCA positivity or negativity is not critical in classifying EGPA [
4]. Ultimately, 25 patients with ANCA-negative GPA and MPA (8 GPA and 17 MPA) were analysed in this study (
Figure 1). The present study was approved by the Institutional Review Board (IRB) of Severance Hospital (Seoul, Korea, IRB no. 4-2020-1071). Given the retrospective design of the study and the use of anonymised patient data, requirements for written informed consent were waived.
Process of classifying AAV
The classification criteria for EGPA, GPA, and MPA were applied in the following order. EGPA was classified when ≥4 of the following six items of the criteria were fulfilled: i) asthma or asthmatic history, ii) sinusitis, iii) peripheral eosinophilia (≥10% of white blood cell counts), iv) migratory and non-fixed lesions in the lungs, v) peripheral neuropathy, and vi) histological features of necrotising vasculitis in small vessels with eosinophil infiltration [
3]. GPA was classified based on three conditions: i) histological features of necrotising vasculitis in small-sized vessels with granuloma formation, ii) histological features of necrotising vasculitis in small vessels without granuloma formation and the presence of surrogate markers for GPA, and iii) no performance of biopsy but the presence of surrogate markers for GPA and ANCA positivity [
2]. MPA was classified based on two conditions: i) histological features of necrotising vasculitis in small vessels without granuloma formation and the absence of surrogate markers for GPA and ii) no performance of biopsy, the absence of surrogate markers for GPA, ANCA positivity, while showing an evidence of renal vasculitis [
2,
11,
12].
Clinical and laboratory data at diagnosis
Demographic information, including age, sex and body mass index, was collected. AAV subtypes, the initial clinical manifestations and AAV-specific indices, such as Birmingham vasculitis activity score (BVAS) version 3 and five-factor score (FFS) were obtained [
13,
14]. Because BVAS for GPA has a different weighting system compared to BVAS version 3, BVAS version 3 was evenly applied to GPA patients to unify the scoring system [
15]. Acute phase reactants including erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) and laboratory results of white blood cell, neutrophil, and platelet count, haemoglobin, as well as serum creatinine and albumin were also reviewed.
ANCAs were detected in the patients using immunofluorescence and ELISA. When a patient had either p-ANCA and/or MPO-ANCA, the patient was regarded as having MPO-ANCA (or p-ANCA), whereas the presence of c-ANCA and/or PR3-ANCA indicated PR3-ANCA (or c-ANCA). The negativity for both tests was considered as ANCA negative.
RESULTS
Baseline characteristics of the study subjects
The median age of patients with ANCA-negative GPA and MPA was 54.0 years and 24% were male. Median body mass index of the patients was calculated as 23.7 kg/m
2. Of 25 patients without ANCA, 8 patients were classified as GPA and 17 as MPA. Among the initial clinical manifestations, renal involvement was the most common (56.0%), followed by cutaneous involvement (56.0%). The median BVAS, FFS, ESR, and CRP levels were 9.0, 1.0, 16.0 mm/h and 1.4 mg/L, respectively. Results of the laboratory investigations are summarised in
Table 1.
Process of classifying ANCA-negative GPA
Five of the 8 patients with ANCA-negative GPA were classified as GPA based on histological features suggesting AAV and granuloma formation (patients 1∼5) [
16]. Three of the 8 patients with ANCA-negative GPA were classified as GPA based on histological features suggesting AAV without granuloma formation and surrogate markers for GPA including refractory otitis media (patients 6 and 7) and cavitary and nodular lesions in lungs (patient 8) (
Table 2) [
2].
Process of classifying ANCA-negative MPA
Fourteen of the 17 ANCA-negative patients were classified as MPA based on histological features suggestive of AAV without granuloma formation and the absence of surrogate markers for GPA (patients 9∼22) [
2]. Three of the 17 patients ANCA-negative MPA (patients 23∼25) exhibited only recurrent cutaneous manifestations without other major organs affected. They were classified as possible MPA based on histological features of necrotising vasculitis in pre-capillary arterioles, capillaries and post-capillary venules without granuloma formation. In addition, there was no evidence of thrombosis, infiltration of neutrophils, eosinophils, or immune deposits. Despite the presence of small vessel vasculitis, because they were not classified as any AAV subtype, immune complex vasculitis, or cutaneous arteritis [
2,
7], they were temporarily classified as possible MPA together with recurrent idiopathic cutaneous leukocytoclastic angiitis (patients 23∼25) (
Table 2).
DISCUSSION
Clinical manifestations, ANCA positivity, and histological features suggestive of MPA or GPA have a paramount importance in the process of classifying GPA and MPA. In comparison, ANCA results and histological findings may not necessarily essential for EGPA diagnosis. For example, the features of asthma, sinusitis, peripheral eosinophilia, migratory lung infiltration, and/or peripheral neuropathy can be used to classify a patient as EGPA regardless of ANCA results and histological features [
3]. However, in cases without pathologic findings in the lung and/or nervous system, histological features should be assessed [
2,
4]. Conversely, in terms of GPA and MPA diagnosis, among the two criteria—histological features and the presence of surrogate markers for AAV—one is required. For example, patients exhibiting necrotising vasculitis in small vessels without granuloma formation may be classified as MPA or GPA based on the presence of surrogate markers for GPA. Meanwhile, in the absence of tissue biopsy, ANCA positivity is a prerequisite in order to be classified as GPA or MPA. Therefore, histologic confirmation should be recommended in patients with ANCA negativity to classify as GPA and MPA [
2].
In the present study, three patients with ANCA-negative MPA (patients 23∼25), who exhibited only cutaneous lesions, including petechiae and purpura, a possibility of three different diseases were initially considered. First, they might have been classified as having cutaneous arteritis because histological features were compatible with necrotising vasculitis that may occasionally affect arterioles. However, the affected vessels were capillaries and post-capillary venules together with pre-capillary arterioles. Therefore, the possibility of cutaneous arteritis was not high. Second, they may simply be classified as having cutaneous leukocytoclastic angiitis, although there were no underlying causes of cutaneous leukocytoclastic angiitis such as infections or malignancies, as described in the Methods section [
17]. However, no infiltration of neutrophils, eosinophils, or immune complex deposits were found [
6,
8]. Alternatively, they may be classified as having cutaneous necrotising vasculitis, but this medical term is not currently recommended [
2,
6]. Lastly, they were temporarily classified as having possible MPA together with recurrent idiopathic cutaneous leukocytoclastic angiitis based on the fact that there was no clear evidence of diseases other than AAV that could explain the patients’ complaints [
1].
Here, we report three patients with MPA who initially exhibited only cutaneous lesions including petechiae and purpura. All of them were female, and the age of the patients ranged from 30∼50. They were suspected to have MPA owing to persistent or recurrent events and were followed up for more than one year. During follow-up, myeloperoxidase-ANCA was detected in patient 23 and 24. They were eventually classified as MPA based on the absence of surrogate markers for GPA, ANCA positivity and clinical features suggestive of renal vasculitis reflected by significant haematuria with proteinuria despite no histological information regarding renal pathology. On the other hand, patient 25 underwent renal biopsy due to significant haematuria and proteinuria and was ultimately classified as MPA according to histological features indicating ANCA-associated pauci-immune glomerulonephritis, and the absence of surrogate markers for GPA. Based on the findings from these patients, although uncommon, it may be possible that idiopathic cutaneous leukocytoclastic vasculitis is a precursor feature of MPA, which implies that a prudent monitoring is required in those with recurrent episodes.
All three patients with ANCA-negative MPA underwent induction therapy with cyclophosphamide or mycophenolate mofetil (MMF) and received maintenance therapy with azathioprine or MMF. To date, these patients have not experienced deterioration in kidney function. The lesson learned from these patients with ANCA-negative GPA or MPA is that the follow-up of these patients with “idiopathic” and “recurrent” cutaneous leukocytoclastic angiitis may be necessary, while it is difficult to define the exact duration of disease, Nevertheless, we believe that the benefit of avoiding irreversible damage to major organs through regular careful follow-up may overcome the inconvenience of repeated examinations and outpatient visits.
To our knowledge, this was the first study to investigate and determine the association between recurrent idiopathic cutaneous leukocytoclastic angiitis and ANCA- negative MPA. Furthermore, we described the clinical implications of recurrent idiopathic cutaneous leukocytoclastic angiitis and emphasised on the necessity of follow-up in this patient population, which may result in earlier clinical suspicion and treatment. However, an important limitation of this study is that the number of patients included in this study is very small. Future investigations with a larger number of patients, exhibiting only recurrent idiopathic cutaneous leukocytoclastic angiitis as the initial symptoms, are warranted to confirm the dynamic classification and validate the necessity of follow-up in these patients.
CONCLUSION
Recurrent idiopathic cutaneous leukocytoclastic angiitis may be associated with ANCA-negative MPA in patients who exhibit cutaneous necrotising vasculitis. In addition, it is recommended for physicians to draw more attention in these patients because it could be a prodromal symptom of MPA.
ACKNOWLEDGMENTS
This research was supported by a faculty research grant of Yonsei University College of Medicine (6-2019-0184) and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (HI14C1324).
REFERENCES
1. Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2013; 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 65:1–11. DOI:
10.1002/art.37715. PMID:
23045170.

2. Watts R, Lane S, Hanslik T, Hauser T, Hellmich B, Koldingsnes W, et al. 2007; Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis. 66:222–7. DOI:
10.1136/ard.2006.054593. PMID:
16901958. PMCID:
PMC1798520.

4. Masi AT, Hunder GG, Lie JT, Michel BA, Bloch DA, Arend WP, et al. 1990; The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum. 33:1094–100. DOI:
10.1002/art.1780330806. PMID:
2202307.

5. Kemna MJ, Damoiseaux J, Austen J, Winkens B, Peters J, van Paassen P, et al. 2015; ANCA as a predictor of relapse: useful in patients with renal involvement but not in patients with nonrenal disease. J Am Soc Nephrol. 26:537–42. DOI:
10.1681/ASN.2013111233. PMID:
25324502. PMCID:
PMC4341475.

6. Tsampau D, Buggiani G, Hercogova J, Lotti T. 2012; Cutaneous necrotizing vasculitis: a rational therapeutic approach. Dermatol Ther. 25:335–9. DOI:
10.1111/j.1529-8019.2012.01480.x. PMID:
22950560.

7. Ozen S. 2017; The changing face of polyarteritis nodosa and necrotizing vasculitis. Nat Rev Rheumatol. 13:381–6. DOI:
10.1038/nrrheum.2017.68. PMID:
28490787.

9. Phatak S, Aggarwal A, Agarwal V, Lawrence A, Misra R. 2017; Antineutrophil cytoplasmic antibody (ANCA) testing: audit from a clinical immunology laboratory. Int J Rheum Dis. 20:774–8. DOI:
10.1111/1756-185X.12926. PMID:
27457216.

10. Moiseev S, Cohen Tervaert JW, Arimura Y, Bogdanos DP, Csernok E, Damoiseaux J, et al. 2020; 2020 international consensus on ANCA testing beyond systemic vasculitis. Autoimmun Rev. 19:102618. DOI:
10.1016/j.autrev.2020.102618. PMID:
32663621.

11. Berden AE, Ferrario F, Hagen EC, Jayne DR, Jennette JC, Joh K, et al. 2010; Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol. 21:1628–36. DOI:
10.1681/ASN.2010050477. PMID:
20616173.

13. Mukhtyar C, Lee R, Brown D, Carruthers D, Dasgupta B, Dubey S, et al. 2009; Modification and validation of the Birmingham Vasculitis Activity Score (version 3). Ann Rheum Dis. 68:1827–32. DOI:
10.1136/ard.2008.101279. PMID:
19054820.

14. Guillevin L, Pagnoux C, Seror R, Mahr A, Mouthon L, Toumelin PL. 2011; The Five-Factor Score revisited: assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Medicine (Baltimore). 90:19–27. DOI:
10.1097/MD.0b013e318205a4c6. PMID:
21200183.
15. Stone JH, Hoffman GS, Merkel PA, Min YI, Uhlfelder ML, Hellmann DB, et al. 2001; A disease-specific activity index for Wegener's granulomatosis: modification of the Birmingham Vasculitis Activity Score. International Network for the Study of the Systemic Vasculitides (INSSYS). Arthritis Rheum. 44:912–20. DOI:
10.1002/1529-0131(200104)44:4<912::AID-ANR148>3.0.CO;2-5.
16. Lutalo PM, D'Cruz DP. 2014; Diagnosis and classification of granulomatosis with polyangiitis (aka Wegener's granulomatosis). J Autoimmun. 48-49:94–8. DOI:
10.1016/j.jaut.2014.01.028. PMID:
24485158.

17. Frumholtz L, Laurent-Roussel S, Lipsker D, Terrier B. 2021; Cutaneous vasculitis: review on diagnosis and clinicopathologic correlations. Clin Rev Allergy Immunol. 61:181–93. DOI:
10.1007/s12016-020-08788-4. PMID:
32378145.

Figure 1
Algorithm of selecting patients with ANCA-negative GPA and MPA. ANCA: antineutrophil cytoplasmic antibody, GPA: granulomatosis with polyangiitis, MPA: microscopic polyangiitis, AAV: ANCA-associated vasculitis, EGPA: eosinophilic GPA.
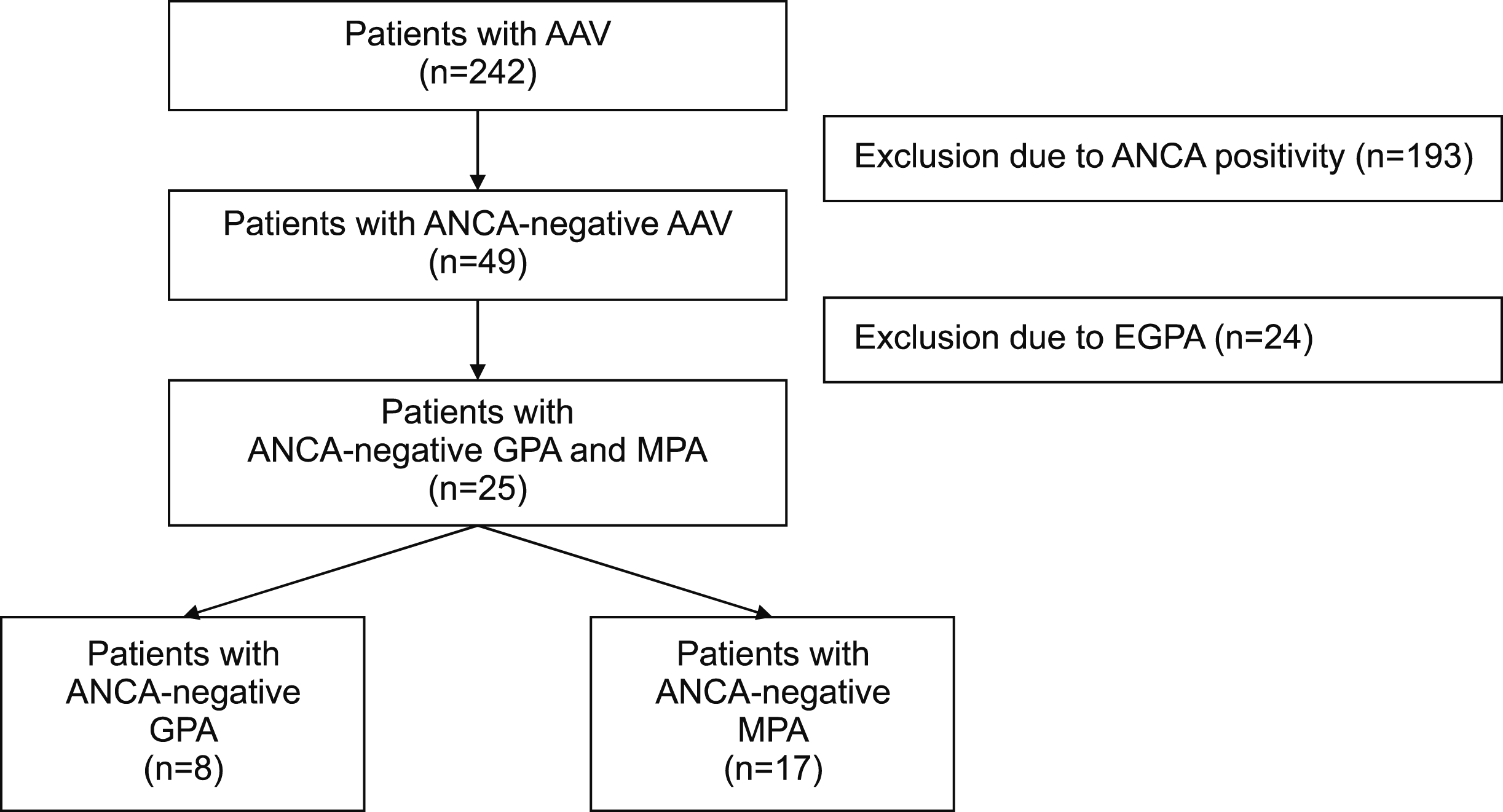
Table 1
Characteristics of patients with ANCA-negative GPA and MPA at diagnosis
|
Variables |
Values |
|
Demographic data |
|
|
Age (yr) |
54.0 (21.5) |
|
Male sex, number (%) |
6 (24.0) |
|
AAV Subtypes, number (%) |
|
|
GPA |
8 (32.0) |
|
MPA |
17 (68.0) |
|
Clinical manifestations, number (%) |
|
|
General manifestations |
9 (36.0) |
|
Cutaneous manifestations |
14 (56.0) |
|
Mucous and ocular manifestations |
4 (16.0) |
|
Otorhinolaryngologic manifestations |
8 (32.0) |
|
Pulmonary manifestations |
7 (28.0) |
|
Cardiovascular manifestations |
7 (28.0) |
|
Gastrointestinal manifestations |
1 (4.0) |
|
Renal manifestations |
14 (56.0) |
|
Nervous systemic manifestations |
8 (32.0) |
|
AAV-specific indices |
|
|
BVAS |
9.0 (12.5) |
|
FFS |
1.0 (1.5) |
|
Acute phase reactants |
|
|
ESR (mm/hr) |
16.0 (33.5) |
|
CRP (mg/L) |
1.4 (25.1) |
|
Laboratory results |
|
|
White blood cell count (/mm3) |
7,025.0 (4,445.0) |
|
Neutrophil count (/mm3) |
4,220.0 (4,740.0) |
|
Haemoglobin (g/dL) |
12.2 (2.6) |
|
Platelet count (×1,000/mm3) |
234.5 (89.3) |
|
Serum creatinine (mg/dL) |
0.7 (0.4) |
|
Serum albumin (g/dL) |
4.0 (1.0) |
Table 2
Process of classifying GPA and MPA in patients without ANCA
|
Number |
AAV
Subtype |
Sex/Age |
Fulfilling the ACR criteria for EGPA |
Histology compatible with GPA |
Histology compatible with MPA with GPA surrogate markers |
Histology compatible with MPA without GPA surrogate markers |
Biopsy sites |
|
1 |
GPA |
M/54 |
No |
Yes |
|
|
Paranasal sinus |
|
2 |
GPA |
F/62 |
No |
Yes |
|
|
Paranasal sinus |
|
3 |
GPA |
F/66 |
No |
Yes |
|
|
Paranasal sinus |
|
4 |
GPA |
F/45 |
No |
Yes |
|
|
Lungs |
|
5 |
GPA |
F/79 |
No |
Yes |
|
|
Lungs |
|
6 |
GPA |
F/58 |
No |
No |
Yes |
|
Kidneys |
|
7 |
GPA |
F/20 |
No |
No |
Yes |
|
Kidneys |
|
8 |
GPA |
M/49 |
No |
No |
Yes |
|
Kidneys |
|
9 |
MPA |
F/62 |
No |
No |
No |
Yes |
Kidneys |
|
10 |
MPA |
M/75 |
No |
No |
No |
Yes |
Kidneys |
|
11 |
MPA |
F/47 |
No |
No |
No |
Yes |
Kidneys |
|
12 |
MPA |
F/69 |
No |
No |
No |
Yes |
Kidneys |
|
13 |
MPA |
F/56 |
No |
No |
No |
Yes |
Kidneys |
|
14 |
MPA |
M/33 |
No |
No |
No |
Yes |
Kidneys |
|
15 |
MPA |
F/50 |
No |
No |
No |
Yes |
Kidneys |
|
16 |
MPA |
M/40 |
No |
No |
No |
Yes |
Kidneys |
|
17 |
MPA |
F/57 |
No |
No |
No |
Yes |
Kidneys |
|
18 |
MPA |
F/69 |
No |
No |
No |
Yes |
Kidneys |
|
19 |
MPA |
M/70 |
No |
No |
No |
Yes |
Kidneys |
|
20 |
MPA |
F/30 |
No |
No |
No |
Yes |
Nerve and skin |
|
21 |
MPA |
F/60 |
No |
No |
No |
Yes |
Paranasal sinus |
|
22 |
MPA |
F/34 |
No |
No |
No |
Yes |
Myocardium and nerve |
|
23 |
MPA |
F/47 |
No |
No |
No |
Possible |
Skin |
|
24 |
MPA |
F/50 |
No |
No |
No |
Possible |
Skin |
|
25 |
MPA |
F/32 |
No |
No |
No |
Possible |
Skin |





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download