INTRODUCTION
Intraductal tubulopapillary neoplasms (ITPNs) of the pancreas and bile duct represent rare premalignant neoplasms that were first recognized by the World Health Organization (WHO) in 2010 [
1]. The first case of ITPN in the pancreas (p-ITPN) was described in 1992 by Shahinian et al. [
2], while the first biliary ITPN (b-ITPN) case was reported in 2010 by Park et al. [
3]. Typically, ITPN presents with jaundice, abdominal pain, and weight loss, resembling pancreatic adenocarcinoma or cholangiocarcinoma, and is most commonly found in patients over 50 years old, with a female overrepresentation. ITPN represents less than 1% of all pancreatic exocrine neoplasms, 3% of pancreatic intraductal neoplasms [
4], and 15% of intraductal neoplasms of the bile duct [
5]. What distinguishes ITPN from other intraductal neoplasms, such as intraductal papillary mucinous neoplasms (IPMN), is their uniquely favorable prognosis.
Given the obscurity of these lesions and the heterogeneity of presentation, we report two cases of ITPN from our center with unusual clinical findings and review the literature on this rare entity.
Go to :

DISCUSSION
ITPN is a rare premalignant entity that is often not diagnosed until after surgical resection. As presented herein, preoperative radiologic, endoscopic, and biopsy studies have proven unreliable for a definitive diagnosis. Standardized guidelines are difficult to establish as the incidence of ITPN is too low for proper evidence-based evaluation.
When ITPN is associated with an invasive component, it is difficult to differentiate it from adenocarcinoma. As of 2018, six cases of the local recurrence of p-ITPN were reported [
9]. There is a significant difference in pathophysiology and prognosis, which makes differentiation at an earlier stage even more imperative. Pancreatic-ITPN is defined as an intraductal, grossly visible, tubule-forming epithelial neoplasm, usually with a minor papillary component, demonstrating high-grade dysplasia and ductal differentiation without mucin production [
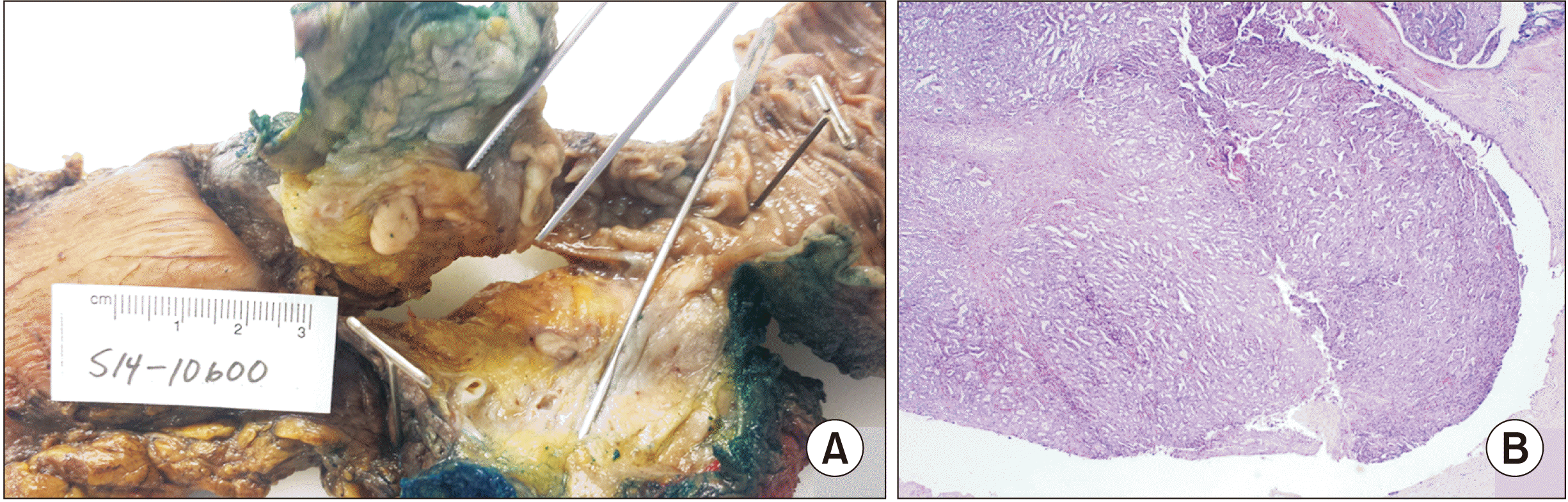
10]. Grossly, these lesions appear solid, occupying a dilated duct and without associated mucin [
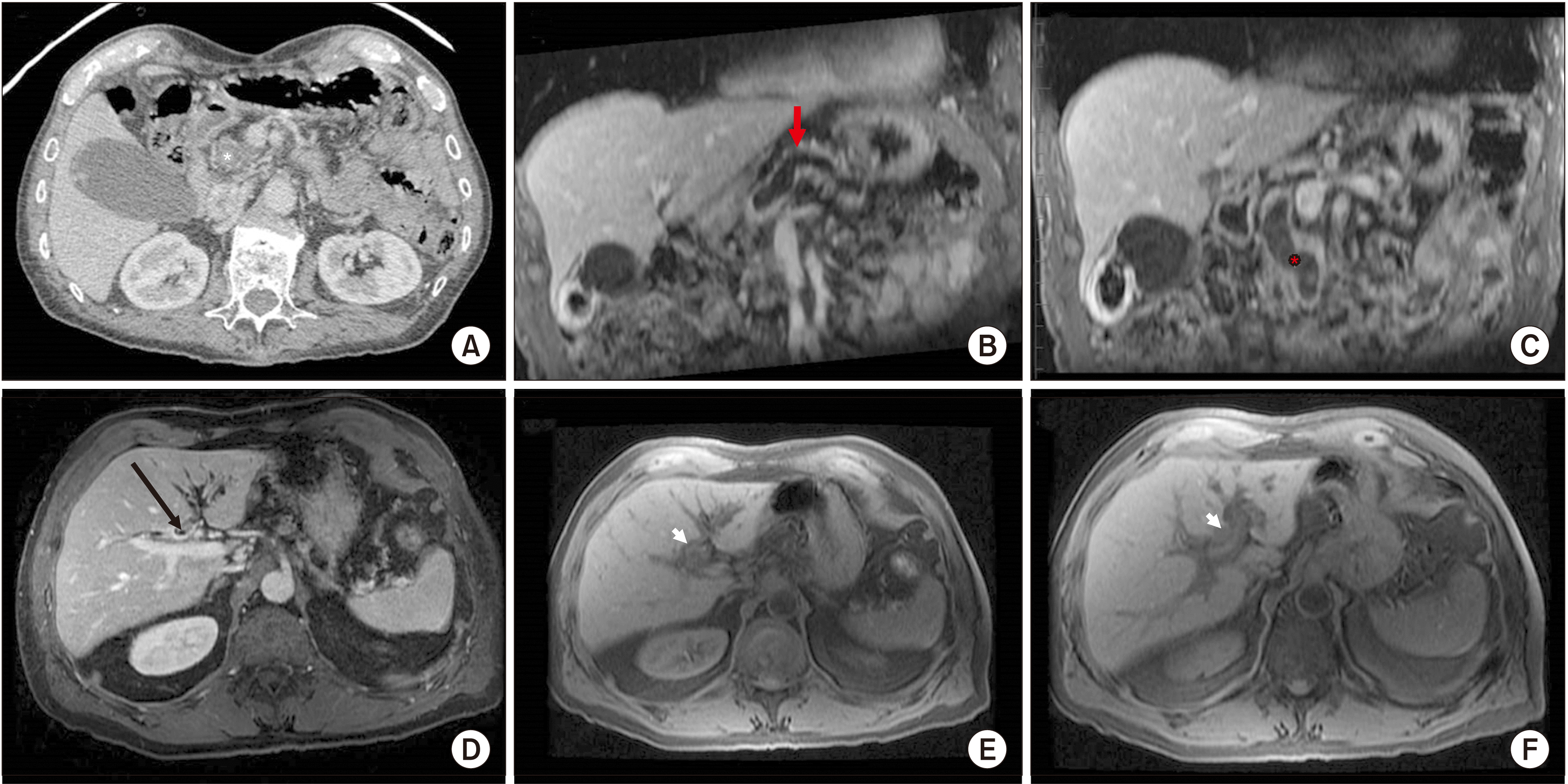
1]. On imaging, p-ITPN typically resembles IPMN or pancreatic adenocarcinoma with low attenuation and a dilated pancreatic duct or double duct sign. An intraductal lesion with the so-called cork in bottle sign may sometimes be detected as well.
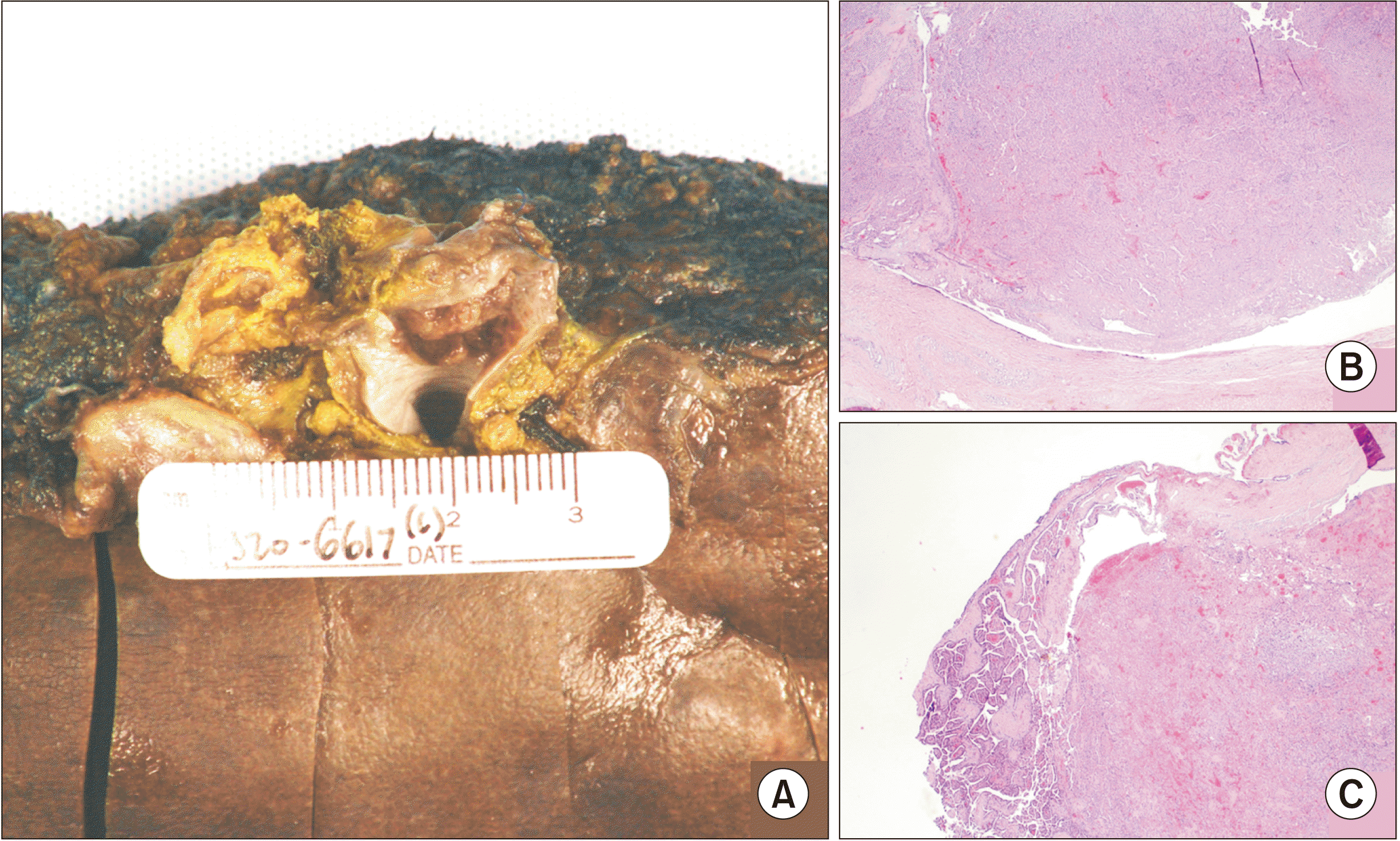
The tumor sites for b-ITPN are intrahepatic (70%), perihilar (20%), and extrahepatic (10%), and appear similar to cystic lesions or cholangiocarcinoma on imaging [
5]. In the largest analysis to date of 20 patients with b-ITPN, invasive carcinoma was identified in 80% of the cases. Despite this, the survival rate was 90% for b-ITPN versus 54% for cholangiocarcinoma [
5]. Similarly, an analysis of 33 patients with p-ITPN showed a 5-year survival rate of 71% for patients without an invasive component; however, 2 of the 33 patients had an invasive cancer [
11]. The pathologic diagnostic criteria for b-ITPN include growth within the intra- or extrahepatic bile duct, preinvasive histology, exophytic growth, and predominantly non-mucinous tubular units with minimal to no papillary growth [
5]. In one review, the 5-year survival rate was 80.7%, compared to 37% in patients with early-stage adenocarcinoma after resection [
12]. Therefore, it is important to consider this when counseling patients before surgery on an ill-defined pancreaticobiliary lesion.
Thus, how can the diagnostic accuracy of preoperative studies be improved? Perhaps a more constructive approach would be to evaluate the radiologic differences between ITPN and IPMN since there is such radiologic overlap between these two entities. Pancreatic ITPN presents a low-density lesion on pancreas-dedicated CT with a dilated pancreatic duct and potentially a “cork in bottle” sign, indicating the presence of a lesion inside the duct. MRCP is the most effective imaging modality to demonstrate this finding. One case series of p-ITPN suggested that EUS was more accurate than cross-sectional imaging [
13]. Two out of the seven patients in this series had intraductal tumors identified on EUS that were not seen on CT or MRI. Despite these identifiers, atypical imaging findings such as diffuse ductal dilation can mimic chronic autoimmune pancreatitis or ductal adenocarcinoma, which may be due to long-standing disease and/or the presence of pancreatic duct stones.
Biliary-ITPN shows ductal dilation and visible intraductal soft tissue with peribiliary liver parenchyma enhancement. In b-ITPN, there is less often a dilated duct downstream given its lack of mucin production, which differentiates it from IPMN, wherein the upstream and downstream ducts are dilated, as was illustrated in our patient [
14]. In a comparison of biliary IPMN and ITPN, the radiologic characteristics of ITPN were an intraductal soft tissue proportion of greater than 60%, peribiliary liver parenchyma enhancement, and mild dilation of the upstream biliary system with no downstream dilation. Typically, there is less liver capsule retraction or parenchymal atrophy compared to IPMN, although in the analysis in this study these differences were not statistically significant [
14]. In addition, the EUS appearance may not be characteristic and the cytologic features of the lesion without gross pathology and histologic examination may be difficult and lead to misdiagnosis.
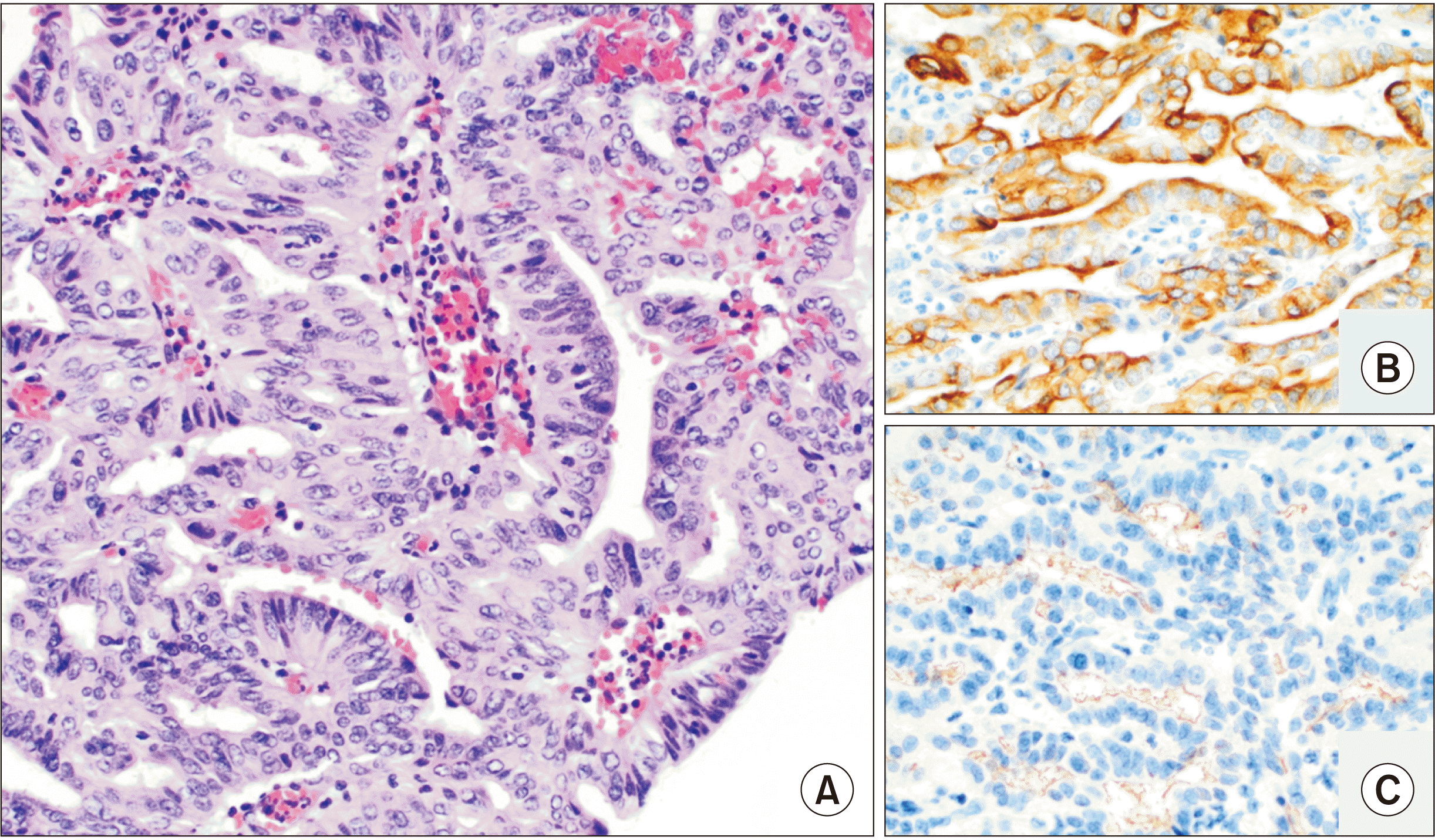
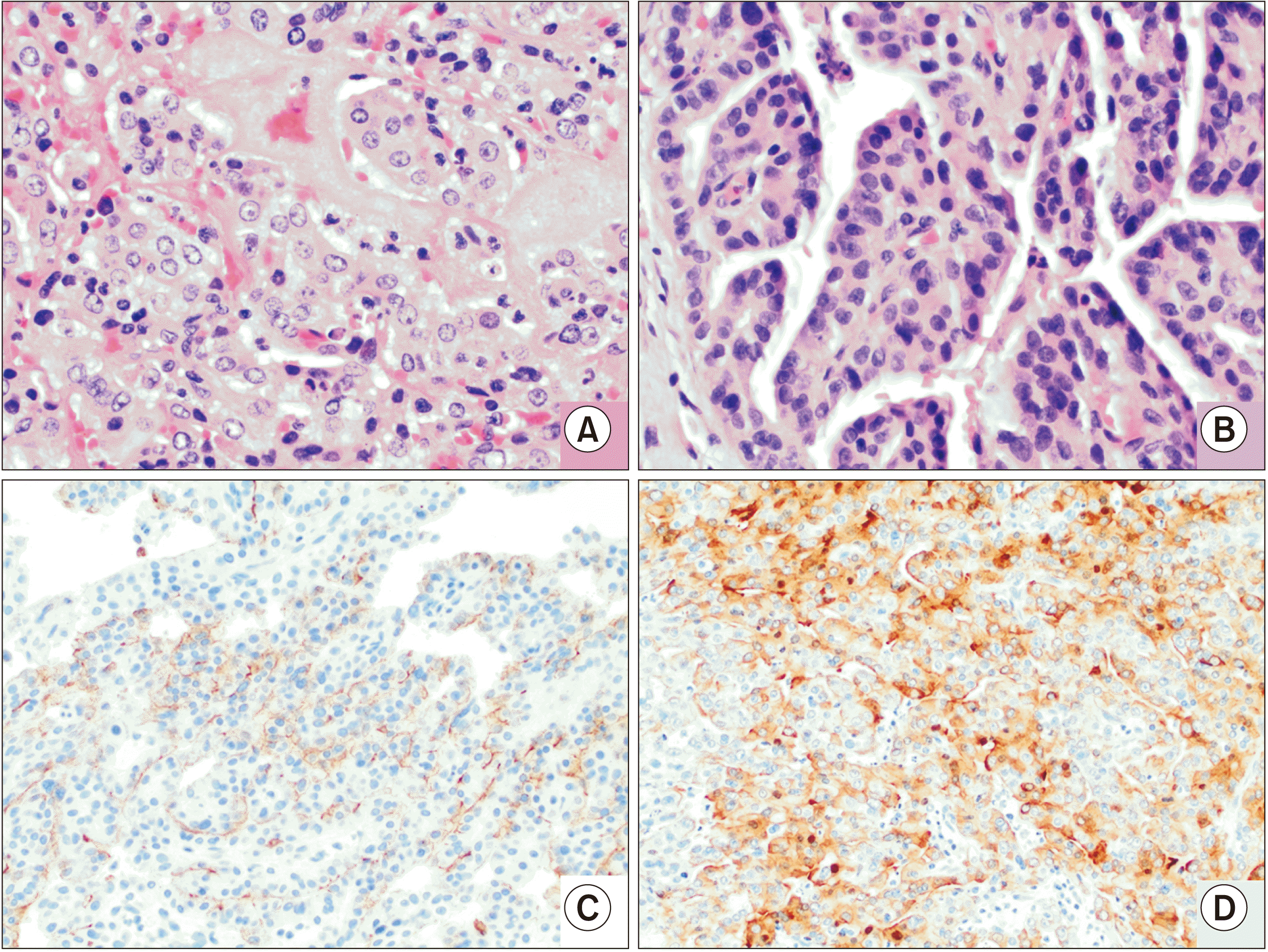
Furthermore, as illustrated in our b-ITPN case, the diagnostic accuracy of preoperative fine-needle aspirate biopsies could be improved. In ITPN, the histologic evaluation reveals a tubulopapillary growth pattern, high-grade nuclear atypia, and the absence of mucin production [
15]. Focal areas of necrosis are frequently identified, and the lesion is often attached to the duct lining by a fibrous stalk. Immunohistochemically, these neoplasms are characterized by the expression of MUC1 and MUC6 versus MUC2 and MUC5AC, which are more often found in mucinous neoplasms, consistent with our findings. While ITPNs of the pancreas and bile ducts represent premalignant lesions, they do not share molecular features with either pancreatobiliary adenocarcinomas or other premalignant lesions, such as IPMN. The most commonly reported mutations of ITPN are PIK3CA, CDKN2A/p16, and TP53, while other commonly reported mutations identified in IPMN and pancreatobiliary adenocarcinomas (KRAS, GNAS, RNF43, BRAF, EGFR, HER2, and beta-catenin) are not typically identified [
1]. In this setting, the cytogenetic analysis of fine-needle aspirates may be of value in ascertaining a diagnosis preoperatively.
Finally, it is important to point out that hepatic resection for hilar cholangiocarcinoma should be limited as much as possible to achieve a curative resection. It has been suggested to perform limited hepatic resection according to the individual tumor extent of each patient [
16]. Because S1 resection is known to be a prerequisite for the curative resection of hilar cholangiocarcinoma [
17-
19], resection of the entire S1 can be the most limited parenchymal-preserving hepatectomy in resection for hilar cholangiocarcinoma.
ITPN of the pancreas and biliary tree are rarely described entities with an elusive diagnostic workup. Resection remains the standard of care, with survival rates that are far superior to other potential hepatobiliary diagnoses. Herein, we discussed two illustrative cases and radiologic identifiers of these pre-malignant tumors to improve future preoperative diagnostic accuracy, which can help better prepare surgeons and patients for the eventual diagnosis.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download