INTRODUCTION
Germ cell tumors (GCTs) are considered as extragonadal if there is no evidence of a primary tumor in the testes or ovaries. Extragonadal GCTs typically arise in midline locations. Their specific sites vary with age. In adults, the most common sites in order of frequency are the anterior mediastinum, the retroperitoneum, and the pineal and suprasellar regions. In infants and young children, sacrococcygeal area and intra cranial GCTs are the most common [
1].
Extragonadal GCTs can be classified as seminomas, non- seminomatous, mature teratomas, and immature teratomas based upon histology. By definition, teratomas derived from at least two of the three embryonic cell layers: ectoderm, mesoderm, and endoderm [
2]. They may be classified as benign or malignant based upon their histological features and cellular mitosis [
3]. Teratomas develop more commonly in the ovaries and testes than in the anterior mediastinum, retroperitoneum, sacrococcygeal region, or the skull [
4]. Less than 1% of cases have been reported in the gastrointestinal tract and liver. Those reported have been more commonly liver teratomas (LTs) that arise from a GCT of the retroperitoneum that involves the liver. LTs are extremely rare. There are only 11 cases reported in adult population until 2018. Isolated liver metastasis of ovarian teratoma is also very rare.
We present a case of a late metachronous recurrence of liver cystic teratoma with gliosis peritonei in a female adult treated by a right extended hepatectomy, along with a literature review.
Go to :

CASE
In August of 2018, a 29-year-old female was referred to our department, Surgical Oncology, Soft Tissue, Bone Tumors and Digestive Tract Tumors, with right upper pain for ten months without other related symptoms. Her past medical history included a left salpingoophorectomy in 1998 due to a mature cyst teratoma without other related management. Physical examination revealed a normal abdomen without a palpable mass. Biochemical studies revealed no alterations for oncological serum markers: alfa-fetoprotein of 1.9 ng/mL, CA19-9 of 1,946 ng/mL, and CA125 of 154 ng/mL. A computed tomography (CT) was performed which revealed a liver mass that involved the IV, V, and VIII Couinaud segments with an heterogeneous density lobulated with gross septa and multiple calcifications (
Fig. 1). These findings were confirmed by magnetic resonance imaging (MRI) (
Fig. 2). A core needle biopsy was performed. Microscopic description revealed fibro connective tissues with fibroblasts fascicles, dystrophic calcification areas, flat stratified and keratinized epithelium, and sebaceous glands as features of mature liver teratoma. Based on previous findings and the diagnosis confirmed by histopathology, a residual liver volume was calculated by CT. It was measured to be 32.33%; with these findings, the surgical team decided to performed a surgical treatment. An exploratory laparotomy was performed with findings of a liver tumor of 150 mm × 120 mm that involved the right lobe, IV, V, and VIII segments that caused extrinsic compression of the inferior vena cava. It was also adhered in its cephalic pole to the right hemi diaphragm. At pelvic recesses, gliosis was also found. No other organs were involved. A right extended hepatectomy was performed without incidents (
Fig. 3–
5). Post-surgical evolution was favorable. She was discharged at seven days after the surgery.
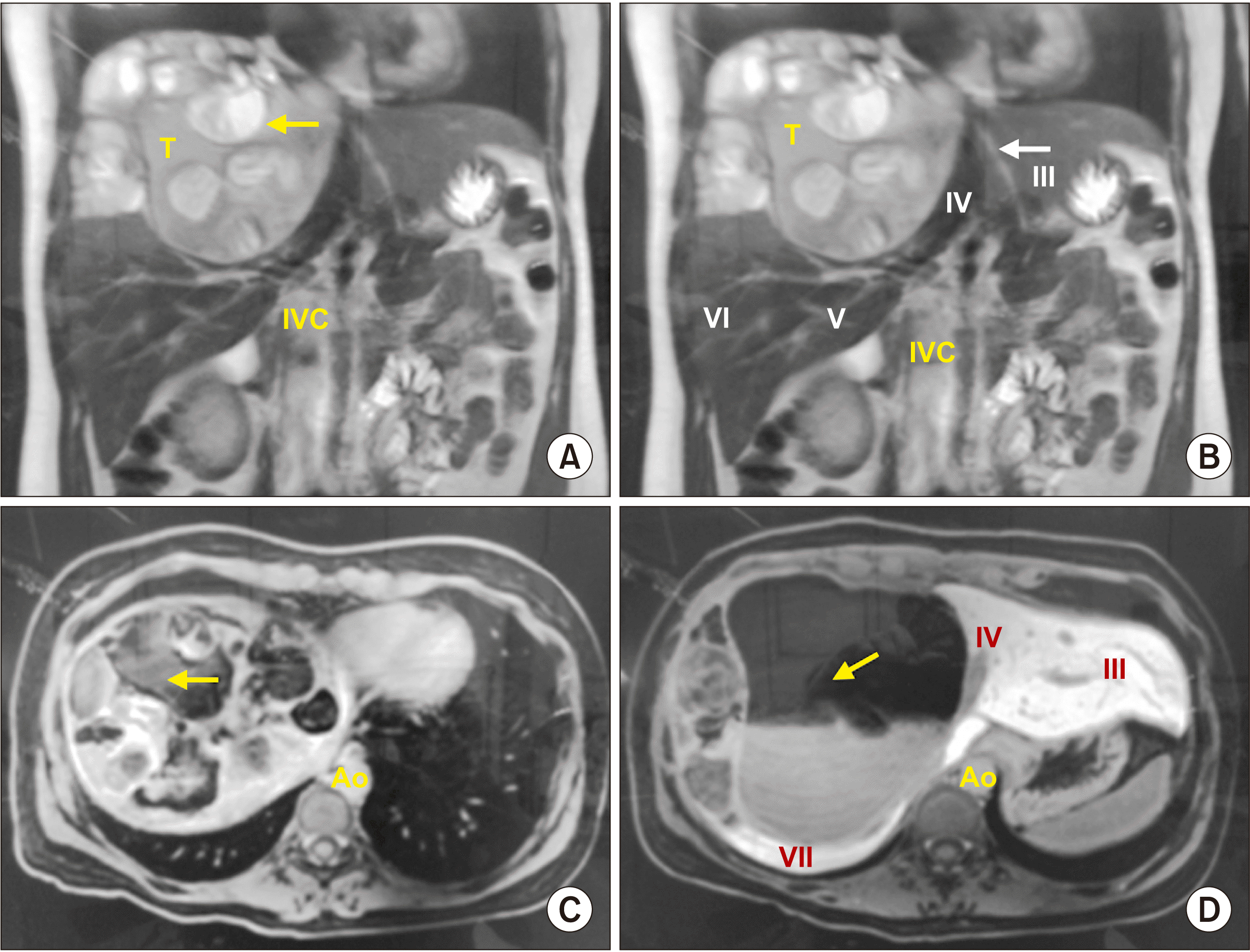 | Fig. 1Magnetic resonance imaging (MRI) demonstrating a liver mass with multiple distinct intensities. Cystic and solid features involving right liver at IV and VIII segments of 160 mm × 180 mm × 90 mm are shown. (A) Coronal MRI in T2 with hypo-intense component suggesting bone calcifications (arrow). (B) Coronal MRI in T2 demonstrating heterogeneous mass in the right liver lobe (IV, V, and VIII), and free liver segments (arrow). (C) Axial MRI in T1 with hypo-intense cystic component (arrow). (D) Axial MRI in T1 with another perspective of cystic component of the tumor (arrow), showing Couinaud liver segments in contact with tumor (roman numeral). IVC, inferior vena cava; Ao, aorta. 
|
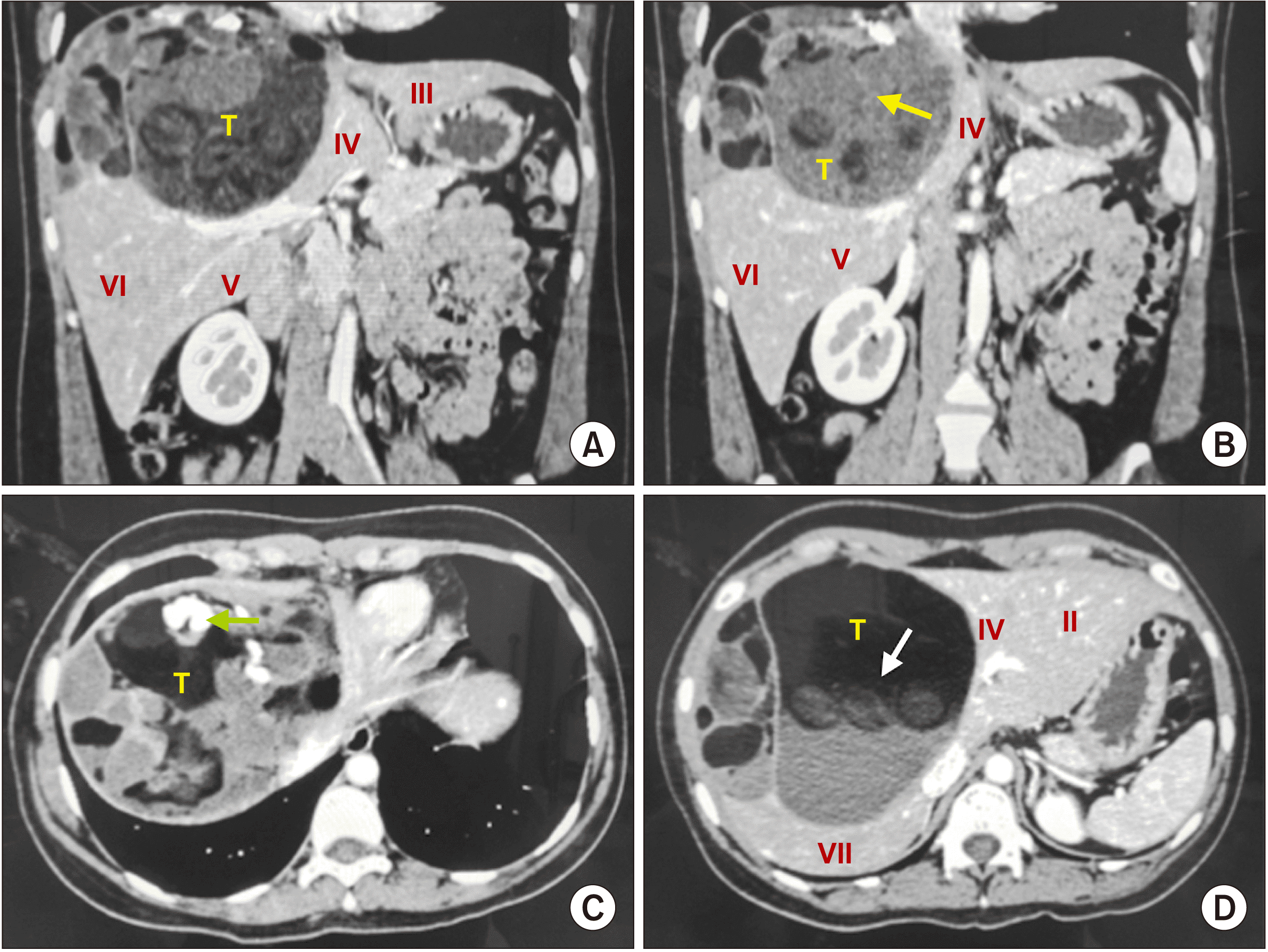 | Fig. 2A 29-year-old female computed tomography in arterial phase. (A) Coronal plane that demonstrating liver mass extension (T), involving the IV, V, and VIII liver segments. Low attenuation in the middle of the mass highly suggestive of fat and hypodense image suggestive of hair (arrow). (B) Low attenuation in the middle of the mass highly suggestive of fat and hypodense image suggestive of hair (arrow). (C) Axial plane showing Rokitansky nodule (arrow). (D) Axial plane where sebaceous tissue is identified (arrow), with features suggestive of liver teratoma. 
|
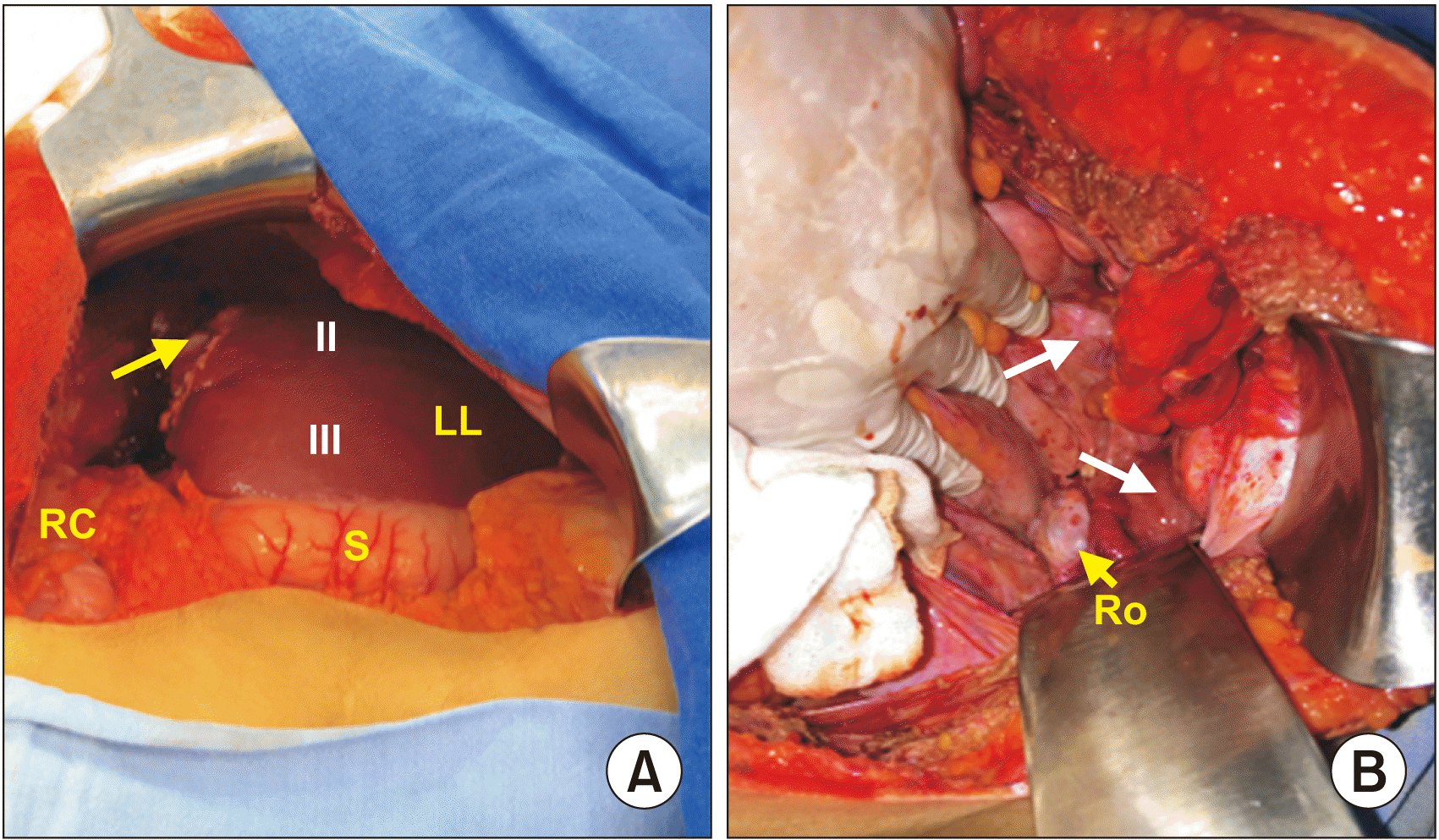 | Fig. 3Trans-operative photo after surgical excision of liver teratoma. (A) Surgical limit of the neoplasia lateral to IV liver segment (arrow) (II, III), residual liver Couinaud segments from left liver (LL), right colon (RC), and stomach (S) are shown. (B) Pearly areas over pelvic recess compatible with gliomatosis peritonei (arrows) and right ovary (Ro). 
|
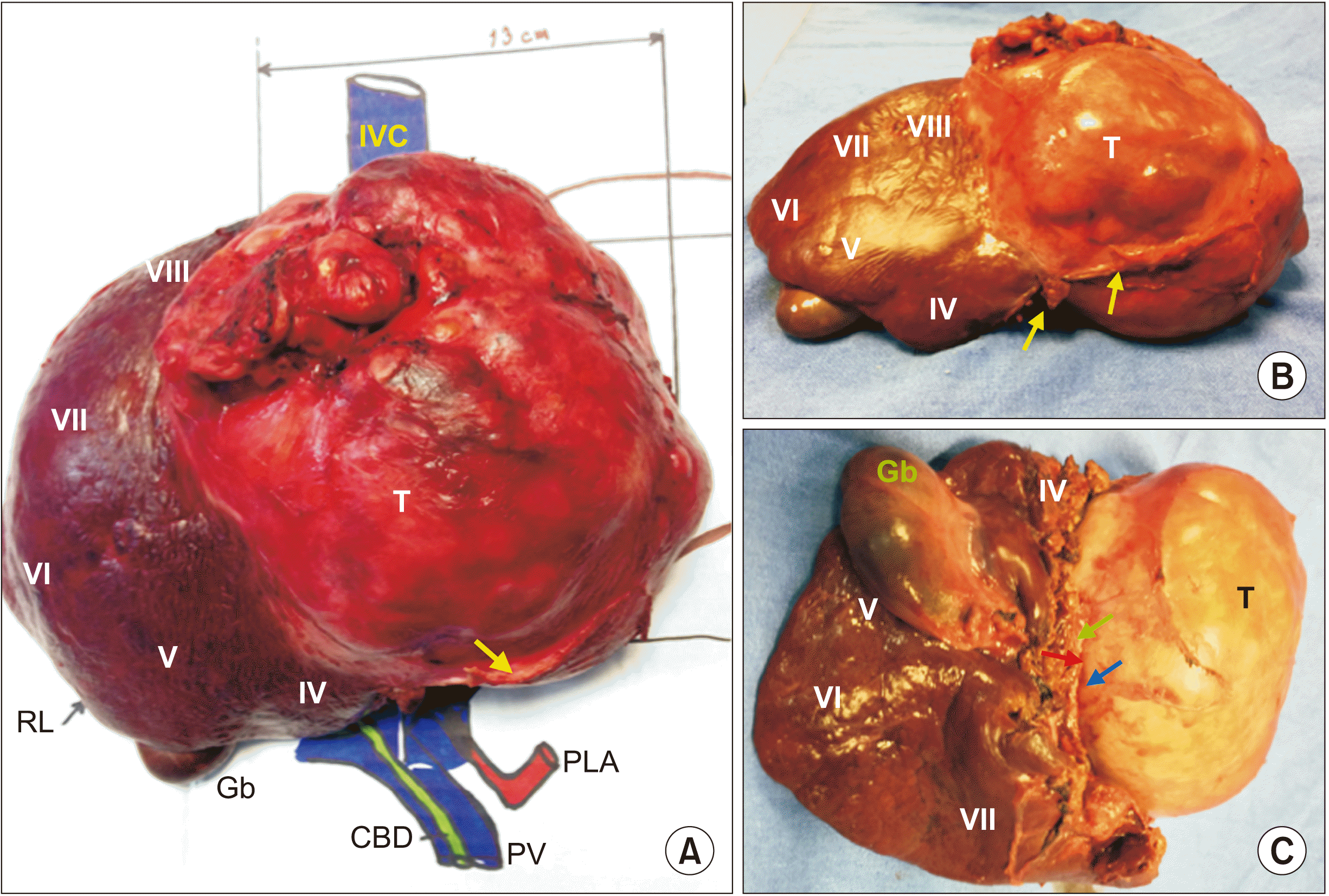 | Fig. 4Surgical specimen product of right extended hepatectomy. (A) Liver teratoma (T) involving IV, V, and VIII liver segments, compression of falciform ligament (arrow), and inferior vena cava (IVC). (B) Upper view demonstrating the extension to IV and VIII liver segments with falciform ligament (arrows). (C) Inferior view showing the tumor extension (T) to the liver segments (IV, VIII), gallbladder (Gb), cystic duct (green arrow), right branch of right liver artery (red arrow), and right branch of portal vein (blue arrow). RL, right liver lobe; CBD, common bile duct; PV, portal vein; PLA, principal liver artery. 
|
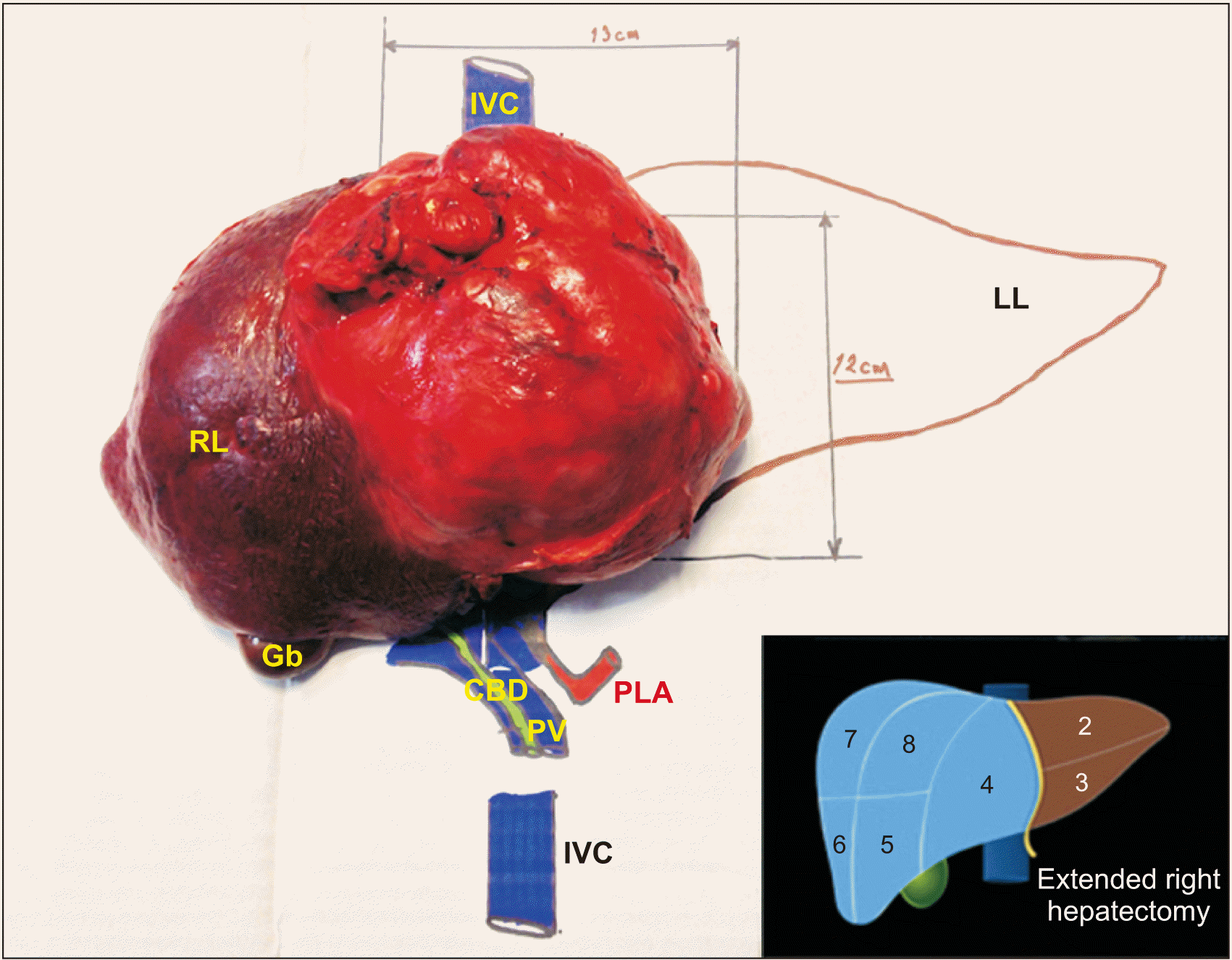 | Fig. 5Specimen mounted in a model after surgical excision to illustrate their anatomical relationships, tumor size, and proper orientation of resected liver segments. IVC, inferior vena cava; RL, right liver lobe; Gb, gallbladder; CBD, common bile duct; PV, portal vein; PLA, principal liver artery; LL, left liver lobe. 
|
The Pathology Department reported a 160 mm × 180 mm × 190 mm neoplasia with multiple cysts, a pasty content interspersed with hair fibers and two pearly looking fronds with hard consistency of 45 mm and 25 mm. Histopathological diagnosis was mature teratoma with heterologous elements and gliosis (
Fig. 6).
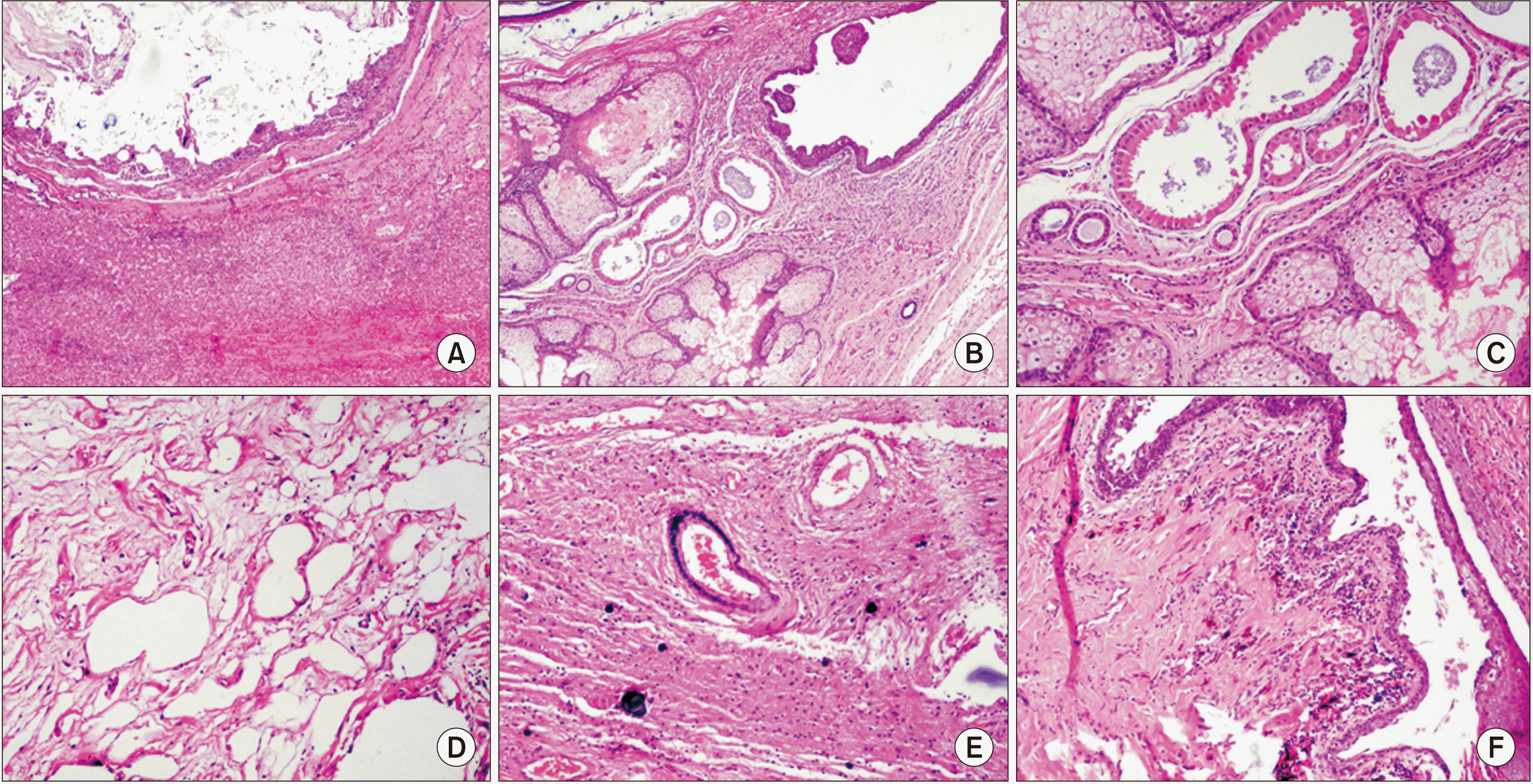 | Fig. 6Photomicrograph with H&E stain. (A) High power field (HPF) 10×. Photomicrograph showing liver parenchyma and cystic lesion with keratin. (B) HPF 20×. Photomicrograph showing mature neuroectodermal elements: sebaceous glands, apocrine metaplasia and epithelia lining of cylindrical ciliated epithelium. (C) HPF 20×. Neuroectodermal elements with rosette formation, ined by crowded basophil cells with hyperchromatic nuclei. (D) HPF 20×. Immature mesenchymal elements: adipose tissue and stroma. (E) HPF 20×. Neuroglia and dystrophic calcification of the psammoma type in gliomatosis peritonei. (F) HPF 10×. Squamous epithelium in transition with immature elements of pigmented neuroepithelium. 
|
Currently, the patient is under surveillance without relapse at the liver. However, a new heterogeneous mass at the right ovary of 50 mm as a possible teratoma was found.
Go to :

DISCUSSION
Most of the reported LTs have been described in infants under 3 years old, exhibiting their primitive germ cell origin. LTs are exceptional in adults. As mature teratomas tend to grow slowly, they are more likely to be diagnosed incidentally while patients are asymptomatic. Clinical symptoms can show due to compression and obstruction of surrounding organs. Occasionally, an acute abdomen can develop as a unique manifestation [
4].
LTs are more common in females without any apparent relation. They are more commonly found in the right lobe liver. LTs are usually well encapsulated tumors that can be easily dissected from the rest of the liver parenchyma. Complete resection of the lesion continues to be the best therapeutic choice [
5].
The pathogenesis of extragonadal GCTs is not clearly defined yet. Until now, two hypotheses have been proposed: The first is that extragonadal GCTs are derived from primordial germ cells that fail to complete the normal migration along the urogenital ridge to the gonadal ridges during embryonal development. This may be due to an abnormality in the primordial germ cell itself or in its microenvironment [
6]. The second hypothesis is that germ cells transformed in the testes might have undergone a reverse migration [
7]. This is supported by genetic data suggesting that extragonadal GCTs and testicular GCTs share a common cell of origin [
8,
9]. However, this hypothesis does not explain biological differences seen among extragonadal GCTs. McKenney et al. [
1] have also argued that extragonadal GCTs could represent metastases developed from an undiagnosed or regressed (“burned out”) primary GCTs in gonads.
Macroscopically, a teratoma usually appears as a well‐demarcated and encapsulated mass with a variegated cut surface, which comprises soft or fleshy areas, cystic areas containing keratinaceous debris or hair shaft, mucoid, or serous materials. Teeth and bone can be present. Histologically, a teratoma is composed of several somatic tissues, with various degrees of maturation and a disorganized distribution. A teratoma is distinct in mature and immature forms, depending on the resemblance to adult or fetal tissues. In immature teratoma, neuroectodermal tissue constituting tubules and rosettes is the most common fetal‐appearing tissue observed. Other fetal tissues including primitive mesenchymal tissue, cartilage, bone, and rhabdomyoblasts are rarely observed [
9]. Teratoma classification is summarized in
Table 1 [
10].
Table 1
Extragonadal teratomas classification [
10]
|
Tumor elements |
Gonzalez-Crussi grading system |
|
Benign teratomas |
|
|
Mature teratoma |
Grade 0: All components are well differentiated |
|
Grade 1: Microscopic foci contain incompletely differentiated tissues, not exceeding 10 percent |
|
Immature teratoma, benign |
Grade 2: Immature tissues make up between 10 and 50 percent of the sample |
|
Grade 3: Over half of the surface is composed of undifferentiated tissues of uncertain metastatic potential |
|
Malignant teratomas |
|
|
With areas of germ cell tumor |
Germinoma
Embryonal Carcinoma
Choriocarcinoma
Yolk sac tumor
Mixed |
|
With nongerminal malignant tumor pattern |
Carcinoma
Sarcoma
Malignant embryonal tumor
Mixed |
|
Inmature teratoma, malignant |
A teratoma that would otherwise be classified as benign inmature teratoma, but which subsequently became metastatic |

Mature teratomas contain well-differentiated histology elements derived from at least two of the three embryonic cell layers: ectoderm, mesoderm, and endoderm. On imaging, they have distinctive features allowing an accurate preoperative diagnosis, on plane films, they can show as a liver masses with calcifications. Occasionally, opacity secondary to a fatty component can be shown. At ultrasonography, there may be a hypoechoic or anechoic component secondary to the cystic portion of the mass. Hyper echogenic foci can be due to calcifications or fat. CT and MRI are helpful for localizing lesions and determining their spatial relationships with surrounding structures. They can also be characterized based on densities within the lesion suggestive of fat, sebaceous material, or cystic elements [
11]. On certain occasions, radiological diagnosis could not be possible and a biopsy with histological examination is required to have a definitive diagnosis [
12].
Ovarian mature teratomas can grow over time, increasing the risk of pain and ovarian accidents. The treatment for mature teratomas is surgical excision which is almost always curative. Partial resection should be limited to patients in whom a complete resection in not technically feasible. There is no evidence-based consensus on the size above which surgical management should be considered. Most studies have used an arbitrary maximum diameter of 5–6 cm amongst their inclusion criteria to offer expectant management. Caspi et al. [
13] have performed a prospective study, to evaluate the progress of ovarian mature cystic teratoma over 3–5 years. They found, that the mean rate of growth of the cyst was 1.5–1.7 mm per year. The ACOG (The American Congress of Obstetricians and Gynecologists) guidelines state that adnexal masses diagnosed during pregnancy appear to have a very low risk for both malignancy and acute complications. Therefore, they may be considered for expectant management [
13,
14].
Mature teratomas are relatively insensitive to both radiation and chemotherapy. For immature ovarian teratomas, current treatment options include an initial staging procedure. Grade 1 and early stage do not require adjuvant treatment. Postoperative surveillance is recommended. GCTs can be managed by postoperative chemotherapy [
11]. In other extragonadal sites (mediastinal immature teratomas), four cycles of cysplatin-based chemotherapy followed by complete surgical resection is suggested if technically feasible, rather than an initial surgery. Etoposide, ifosfamide, and cisplatin (VIP) are preferred rather than bleomycin, etoposide, and cisplatin (BEP) because of their risk of pulmonary toxicity.
LTs are extremely rare. To date, there are few cases reported in adult population. After a bibliographic research in PubMed, we found 11 cases of liver teratoma up to 2018. The treatment consisted of anatomic hepatectomy in four of them, enucleation in three, partial excision in two, and surveillance in only one case. Complete paper and patient data could not be obtained for one case. Thus, only 10 cases were assessed. Results are shown, in
Table 2 [
12-
23].
Table 2
Liver teratoma characteristics of reported cases from 2003 to 2018
|
Author |
Year |
Age (yr) |
Involved segments |
Treatment |
Size (cm) |
Follow-up (mon) |
|
Cöl [15] |
2003 |
21 |
VII, VIII |
Right hepatectomy |
18 |
4 |
|
Martin et al. [23] |
2003 |
53 |
VII, VIII (adjacent) |
Enucleation |
8 |
NR |
|
Rahmat et al. [16] |
2006 |
46 |
II, III |
Left hepatectomy |
8 |
NR |
|
Silva et al. [17] |
2007 |
27 |
VII |
Right hepatectomy |
9 |
26 |
|
Certo et al. [18] |
2008 |
27 |
V (adjacent) |
Partial hepatectomy |
8 |
NR |
|
Karlo et al. [19] |
2009 |
59 |
VIII (adjacent) |
Enucleation |
15 |
NR |
|
Madan et al. [20] |
2010 |
34 |
VII, VIII (adjacent) |
Enucleation |
13.5 |
NR |
|
Harris and De Simone [21] |
2011 |
57 |
VII, VIII (adjacent) |
Surveillance |
4.5 |
NR |
|
Gupta et al. [22] |
2013 |
4 |
I (adjacent) |
Partial excision |
4 |
NR |
|
Ramkumar et al. [12] |
2018 |
65 |
V, VIII |
Debulking |
12 |
1 |

Gliomatosis peritonei (GP) is an unusual condition often associated with ovarian immature teratomas, it is characterized by the presence of mature glial tissue on peritoneum [
24]. In other rare cases, it shows ventriculoperitoneal shunt [
25,
26]. Despite GP extension, it is not associated with a worse prognosis [
27]. Nevertheless, malignant glial transformation has been reported. GP is considered as grade 0 teratoma. Its management is conservative. The presence of GP might indicate mature or immature teratoma metastasis [
28].
Although GP is associated with capsular rupture of ovarian teratoma in most cases, there is genetic evidence showing that it could have a different genetic identity, thus generating a discussion about whether GP is an associated or an independent phenomenon [
29].
Recurrence of ovarian mature cyst teratomas is very rare after surgical excision (4.2%). In a study of 382 patients, young age (< 30 years), large cyst (> 8 cm in diameter), and bilaterality were significant risk factors for recurrence [
30].
Neoplasm metastasis originating from a primary tumor has one type of tissue which undergoes malignant transformation. It is entirely different in the case of teratomas as primary tumors, due to the characteristics of teratomas which are composed of several tissues, either from one or more germ layers. They are not present in the region of origin. A single tissue in a benign teratoma may undergo malignant transformation and this one tissue, either carcinomatous or sarcomatous, may metastasize [
31].
A search was carried out in PubMed from 1920 to 2021 to try to explain the hepatic relapse under the name of mature teratoma liver “metastasis”. This search revealed very little information about metastasis in the liver of mature teratoma as observed in our patient.
Kommoss et al. [
32] have described a case of rupture of an ovarian mature cystic teratoma causing intraperitoneal spillage of tumor contents and, leading to recurrence as cystic masses in the liver and colon 17 years after. On the other hand, there is only one reported case of recurrence as a non-intrahepatic perihepatic mass shown as, a mature teratoma with GP [
33], as in our case.
Okino et al. [
34] have reported a case of mature teratoma and its development in the diaphragm 34 years after removing the primary one in the left ovary, in which the metachronic term is included as the best possibility to distinguish it from recurrence, explaining the following: with a 34-year interval from the excision of the primary tumor, the presence of the 3 primitive germ layers and, absence of gliomatosis (unlike our case), indicate the final diagnosis as “mature cystic teratomas showing multicentric and metachronous developments at multiple sites.”
Li et al. [
35] described a rare case of giant liver metastasis, very similar to ours. However, their occurred in the context of growing teratoma syndrome in which condition are characterized by persistence or the development of enlarging masses following an adjuvant chemotheraphy during treatment for GCTs.
In conclusions, our case demonstrates an extremely uncommon late metachronous relapse in liver localization of mature teratoma and the surgical challenge due to its size, parenchymal extension, and presence of GP. Surgical excision is the treatment of choice. This entity has an excellent prognosis.
Go to :











 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download