Abstract
Although angiomyolipoma (AML) is commonly found in the kidney, its appearance in the liver is rare. The first hepatic AML was reported by Ishak in 1976. Since then, there have been various reports of AML. An AML is a tumour affecting adipocytes, muscle tissues, and blood vessels. Hepatic AML has been associated with tuberous sclerosis. Its spontaneous occurrence has also been noticed. It can have a varied presentation with some being asymptomatic, others presenting with a dull aching pain, while some with disastrous consequences due to rupture and torrential bleeding intra-abdominally. Herein, we present a case of a 47-year-old female with a large hepatic AML having an internal hemorrhage that caused changes in hepatic arteries. In our case, magnetic resonance imaging was unable to establish a diagnosis. Intraoperatively, AML caused dilatation and engorgement of vessels around the porta. Immediately post resection, vessel dilatation and engorgement were reduced on table. Another notable feature was that these changes caused no intra-operative or post-operative hemodynamic changes. We report a case of a huge hepatic AML with internal hemorrhage associated with perihepatic vascular changes having a successful surgical treatment.
The hepatic parenchyma is the host for a variety of tumors, both benign and malignant [1]. Hepatocellular carcinoma (HCC), cholangiocarcinoma, hepatocellular adenoma, and focal noduar hyperplasia are some common tumours found in the liver and frequently discussed in the literature [2]. On the other hand, hepatic angiomyolipoma (AML) is a relatively rare hepatic tumour [3]. Although AMLs are commonly found in the kidney, its appearance in the liver is rare [4]. The first hepatic AML was reported by Ishak in 1976. Since then, there have been various reports of AML [5]. An AML is a tumour affecting adipocytes, muscle tissues, and blood vessels [6]. It commonly affects patients with tuberous sclerosis [7]. Hepatic AML poses a challenge for diagnosis as it mimics HCC [4]. It is commonly located in the right lobe. It is rarely present with an internal hemorrhage. Herein, we present a case of a 47-year-old female with a large hepatic AML with internal hemorrhage causing changes in hepatic arteries.
A 47-year-old female visited the hospital with a sudden abdominal pain and swelling on the right side of the abdomen. The patient had no co-morbidities or no previous history of a liver disease. At another hospital, she underwent abdomen ultrasonography. She was diagnosed with mucinous cystic neoplasm of the liver. More than two litres of blood were aspirated from the cyst. She received blood transfusion. Subsequent transarterial embolization was carried out to tackle the bleeding. However, her pain persisted. A follow-up computed tomography was suggestive of a differential diagnosis of a 19-cm hemangioma with internal hemorrhage or mucinous cystic neoplasm along with venous congestion in the right liver (Fig. 1).
She was then offered a curative surgical treatment. On blood investigations, all her results were within normal limits except a slightly raised alkaline phosphatase level. An open extended right hemihepatectomy was planned. During the surgery, a huge cystic hepatic tumor with dilated and engorged arteries and portal veins at the porta was observed (Fig. 2). An intraoperative ultrasound was carried out. It showed a cystic lesion containing septations along with solid components. Using an anterior approach, extended right hemihepatectomy including middle hepatic vein was performed. Due to engorged vessels, division of the right Glisson pedicle was difficult. A decision was made to use vascular staplers. After completing liver resection, the right liver was mobilized and removed without rupture of the tumor. A Jackson Pratt drain was inserted. After specimen retrieval, the remnant common and left hepatic artery engorgement were slightly reduced. The surgery lasted 150 minutes with an estimated blood loss of 350 mL. The patient was transferred to the intensive care unit considering the possibility of hemodynamic changes due to the presence of engorged arteries. Post-surgery, the patient remained vitally stable without any hemodynamic instability or immediate postoperative complications. She was discharged on the 6th postoperative day.
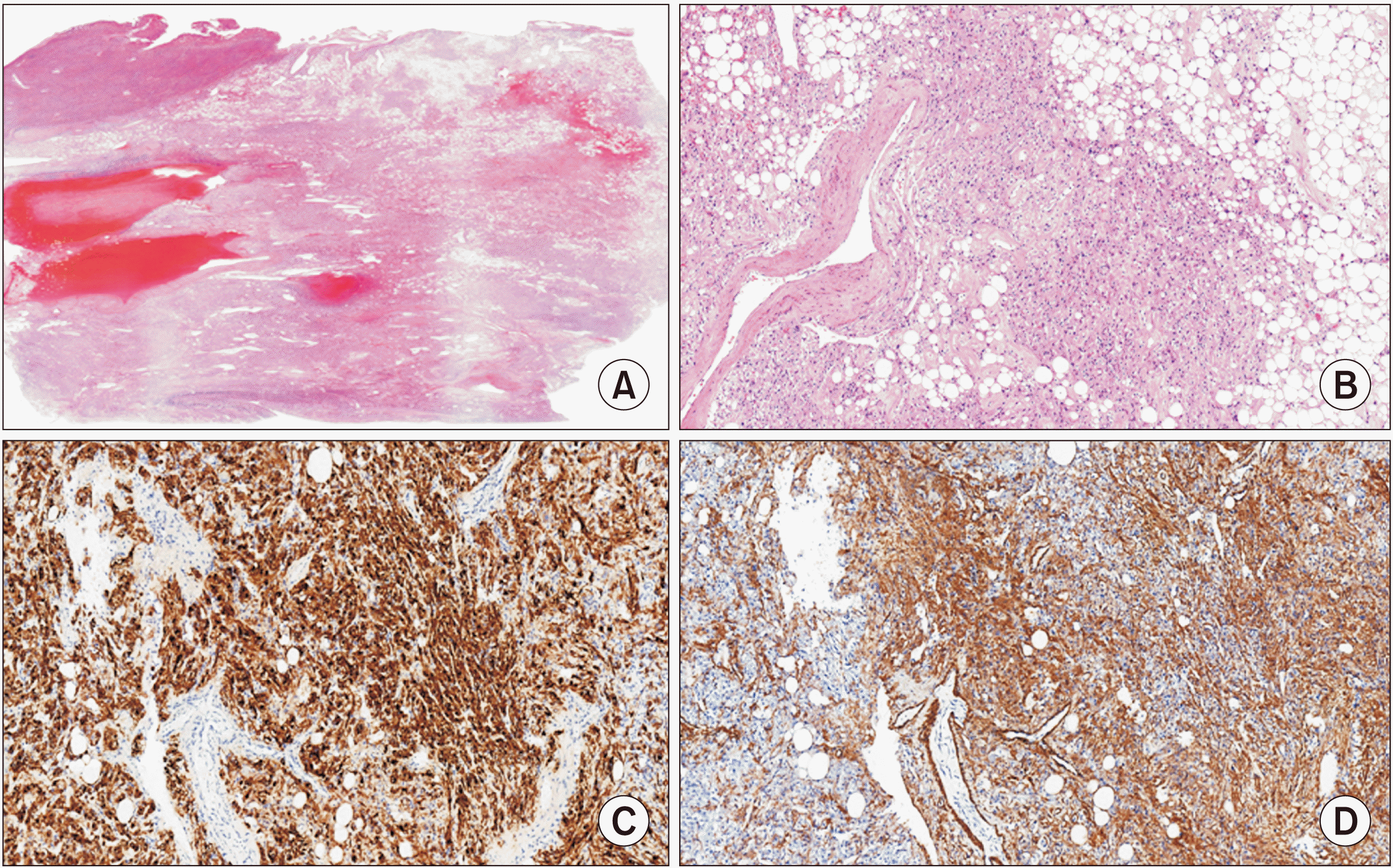
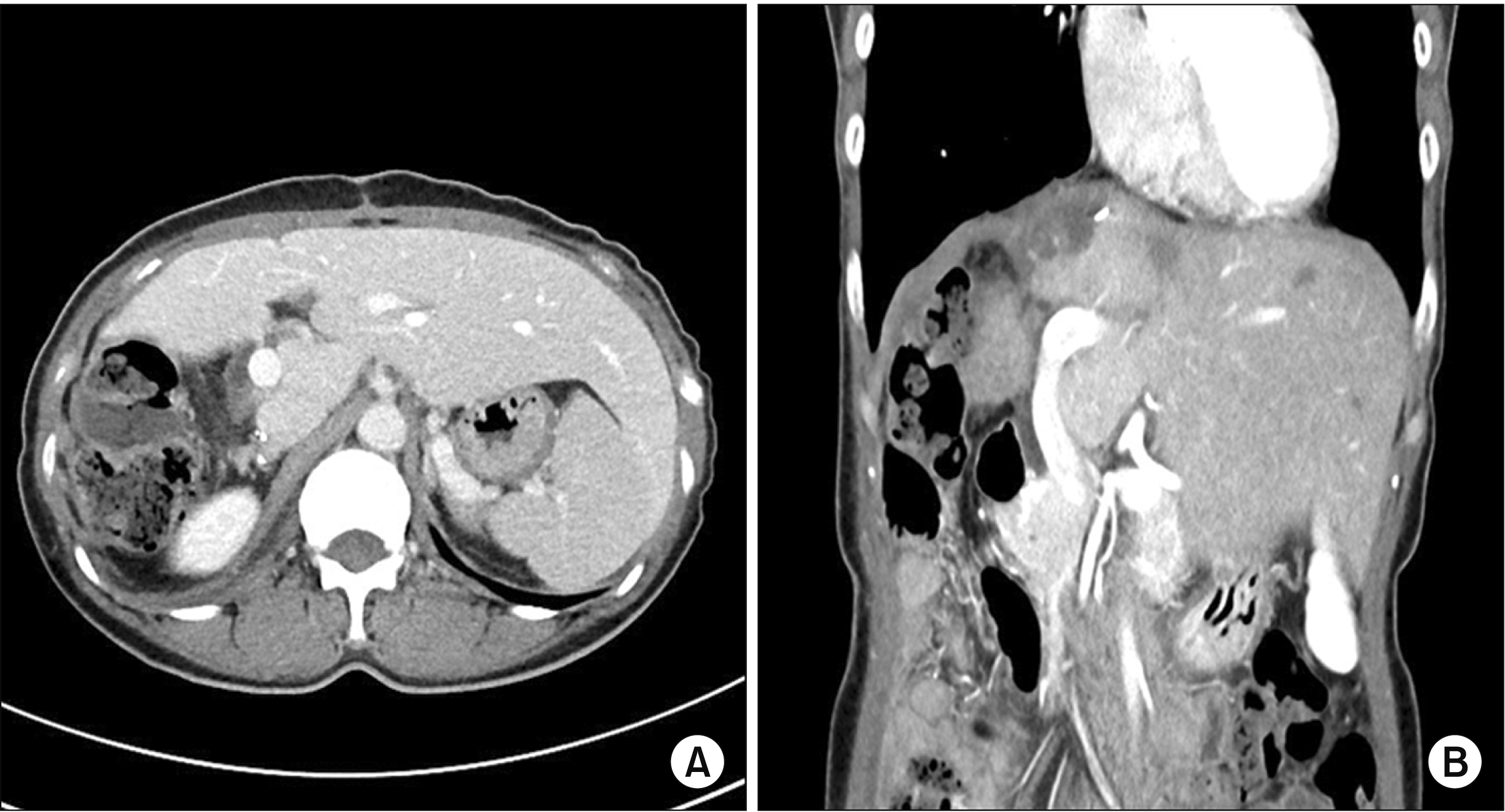
Histopathology showed a 15 cm × 13 cm × 6 cm hepatic AML with a combination of internal hemorrhage and 60% of epithelioid cells. Mild sinusoidal dilatation with no specific abnormality in microscopic arteries was found (Fig. 3). On follow-up at 6 months after the surgery, the patient was doing well with normal liver function. She had no complications. A follow-up computed tomography showed well regenerated remnant left liver and a further reduction of perihepatic arteries (Fig. 4).
Hepatic AML is a rare tumour without much information about it. It is commonly found in young adult females, although there have been some case reports in males [8]. Hepatic AML has been associated with tuberous sclerosis, although its spontaneous occurrence has also been noticed. It can have a varied presentation with some being asymptomatic, others presenting with a dull aching pain, while some with disastrous consequences due to rupture and torrential bleeding intra abdominally [9]. There are various types of AMLs based on the predominant component, including the myoma type with smooth muscles, the lipoma type with adipose tissue, the hemangioma type with vascular tissue, the epithelioid type with epithelial cells, and the hybrid type which is the most common type that constitutes equal amounts of each component [10]. Diagnosis of AML is challenging. One of the reasons for this is the percentage of adipocytes varies from 5% to 90% in different AMLs [11]. Therefore, it may mimic other benign tumours like it did in our case, where magnetic resonance imaging (MRI) was suggestive of haemangioma or mucinous cystic neoplasm due to the appearance of predominance of vascular tissue. However, pathology showed a 60% epithelioid component. Surgery is the primary mode of management. Operating on an AML is controversial due to its benign nature. However, there exists a risk of malignancy as well as a risk of rupture. Ding et al. [12] have developed the following criteria for surgery: symptomatic patients, tumours greater than 6 cm in size or showing a tendency to grow, tumours showing extrahepatic growth and risk of rupture, and equivocal findings when a definitive diagnosis cannot be established. Pathologically, AML is predominantly benign, although some can have malignant potential.
In our case, MRI was unable to establish a diagnosis. Intraoperatively, AML caused dilatation and engorgement of vessels around the porta. Immediately post resection, the vessel dilatation and engorgement showed reductions on table. Another notable feature was that these changes caused no intra-operative or postoperative hemodynamic changes. The real reason for this occurrence was debatable. It was unclear whether dilation and engorgement of both arteries and veins were due to an arteriovenous malformation in addition to pure vessel involvement or due to mass effect by sudden internal hemorrhage. Pathology showed sinusoidal dilatation. However, there was no dilatation in microscopic arteries. Further investigations are needed to study vascular changes associated with hepatic AML.
In conclusion, although hepatic AML is a tumour with a rare occurrence, it can have disastrous consequences such as internal hemorrhage in a patient. The exact changes in blood vessels need to be studied further. We report a case of a huge hepatic AML with internal hemorrhage associated with perihepatic vascular changes having a successful surgical treatment.
REFERENCES
1. Roncalli M, Sciarra A, Tommaso LD. 2016; Benign hepatocellular nodules of healthy liver: focal nodular hyperplasia and hepatocellular adenoma. Clin Mol Hepatol. 22:199–211. DOI: 10.3350/cmh.2016.0101. PMID: 27189732. PMCID: PMC4946404.

2. Bruix J, Han KH, Gores G, Llovet JM, Mazzaferro V. 2015; Liver cancer: approaching a personalized care. J Hepatol. 62(1 Suppl):S144–S156. DOI: 10.1016/j.jhep.2015.02.007. PMID: 25920083. PMCID: PMC4520430.

3. Guidi G, Catalano O, Rotondo A. 1997; Spontaneous rupture of a hepatic angiomyolipoma: CT findings and literature review. Eur Radiol. 7:335–337. DOI: 10.1007/s003300050162. PMID: 9087353.

4. Blokhin I, Chernina V, Menglibaev M, Kalinin D, Schima W, Karmazanovsky G. 2018; Giant hepatic angiomyolipoma: a case report. BJR Case Rep. 5:20180072. DOI: 10.1259/bjrcr.20180072. PMID: 31131134. PMCID: PMC6519506.

5. Huber C, Treutner KH, Steinau G, Schumpelick V. 1996; Ruptured hepatic angiolipoma in tuberous sclerosis complex. Langenbecks Arch Chir. 381:7–9. DOI: 10.1007/BF00184248. PMID: 8717168.

6. Kim SH, Kang TW, Lim K, Joh HS, Kang J, Sinn DH. 2017; A case of ruptured hepatic angiomyolipoma in a young male. Clin Mol Hepatol. 23:179–183. DOI: 10.3350/cmh.2016.0027. PMID: 28449573. PMCID: PMC5497672.

7. Galant J, Martí-Bonmatí L, Ripollés T, Martinez-Rodrigo J, Ferrer MD. 1995; Hepatic manifestations of tuberous sclerosis studied by US and CT. Eur Radiol. 5:486–491. DOI: 10.1007/BF00208339.

8. Lee SJ, Kim SY, Kim KW, Kim JH, Kim HJ, Lee MG, et al. 2016; Hepatic angiomyolipoma versus hepatocellular carcinoma in the noncirrhotic liver on gadoxetic acid-enhanced MRI: a diagnostic challenge. AJR Am J Roentgenol. 207:562–570. DOI: 10.2214/AJR.15.15602. PMID: 27248975.

9. Yang L, Xu Z, Dong R, Fan J, Du Y, Zhang Y, et al. 2013; Is surgery necessary for patients with hepatic angiomyolipoma? Retrospective analysis from eight Chinese cases. J Gastroenterol Hepatol. 28:1648–1653. DOI: 10.1111/jgh.12289. PMID: 23731017.
10. Du S, Li Y, Mao Y, Sang X, Lu X, Wang W, et al. 2012; Diagnosis and treatment of hepatic angiomyolipoma. Hepatobiliary Surg Nutr. 1:19–24. DOI: 10.3978/j.issn.2304-3881.2012.07.02. PMID: 24570900. PMCID: PMC3924623.
11. Goodman ZD, Ishak KG. 1984; Angiomyolipomas of the liver. Am J Surg Pathol. 8:745–750. DOI: 10.1097/00000478-198410000-00003. PMID: 6496843.

12. Ding GH, Liu Y, Wu MC, Yang GS, Yang JM, Cong WM. 2011; Diagnosis and treatment of hepatic angiomyolipoma. J Surg Oncol. 103:807–812. DOI: 10.1002/jso.21814. PMID: 21283992.

Fig. 1
Preoperative images. Huge cystic tumor or hemorrhage in the right liver on computed tomography (CT) (A), suspected mural nodules inside the cystic tumor on magnetic resonance imaging (B), engorged hepatic artery and huge tumor on angiogram (C), and persistent cystic and solid huge tumor on immediate preoperative CT scan (D).
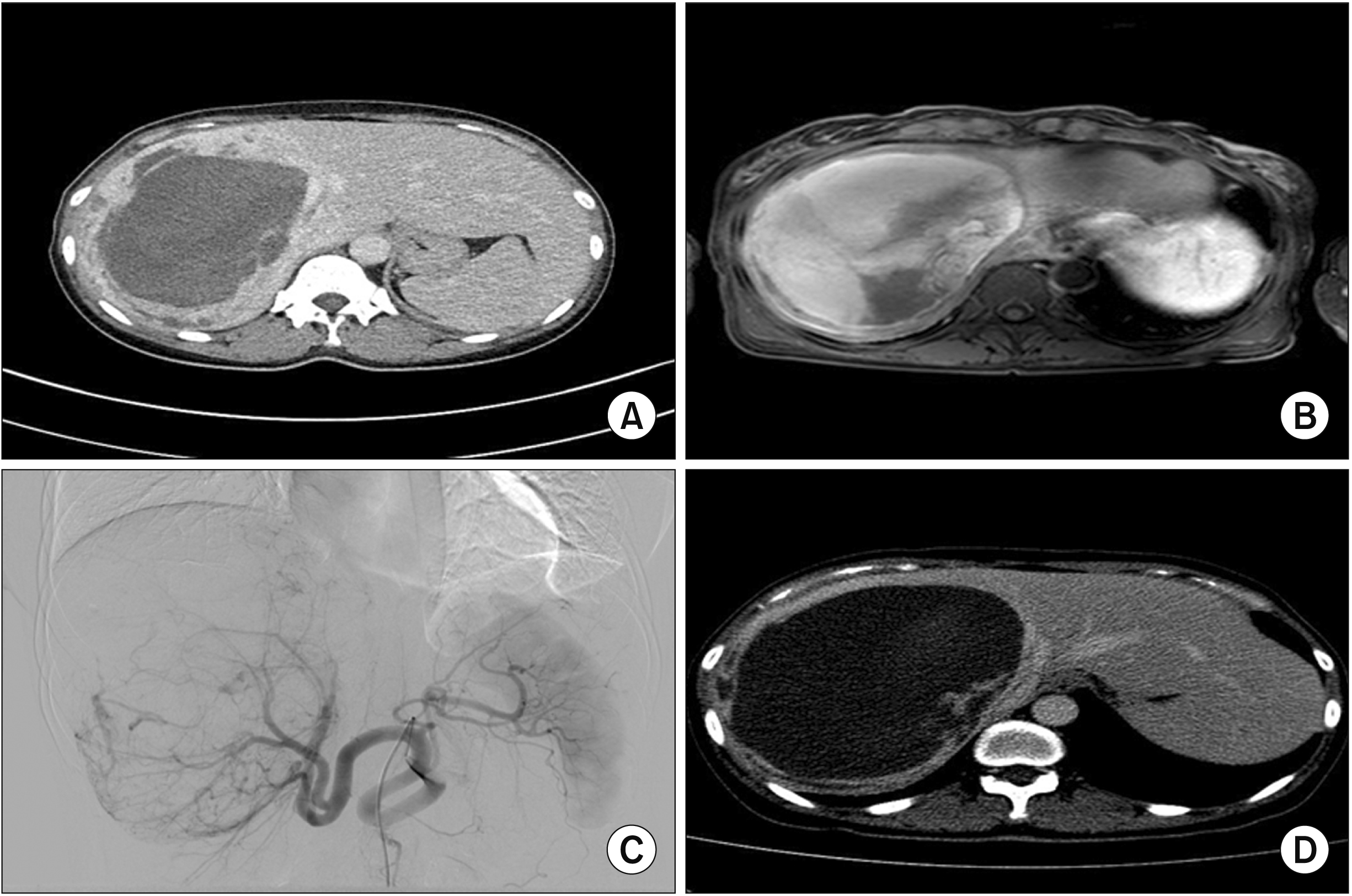
Fig. 2
Intraoperative images showing engorgement of vessels at the porta hepatis (A) and slight reduction of engorgement after right hepatectomy (B).
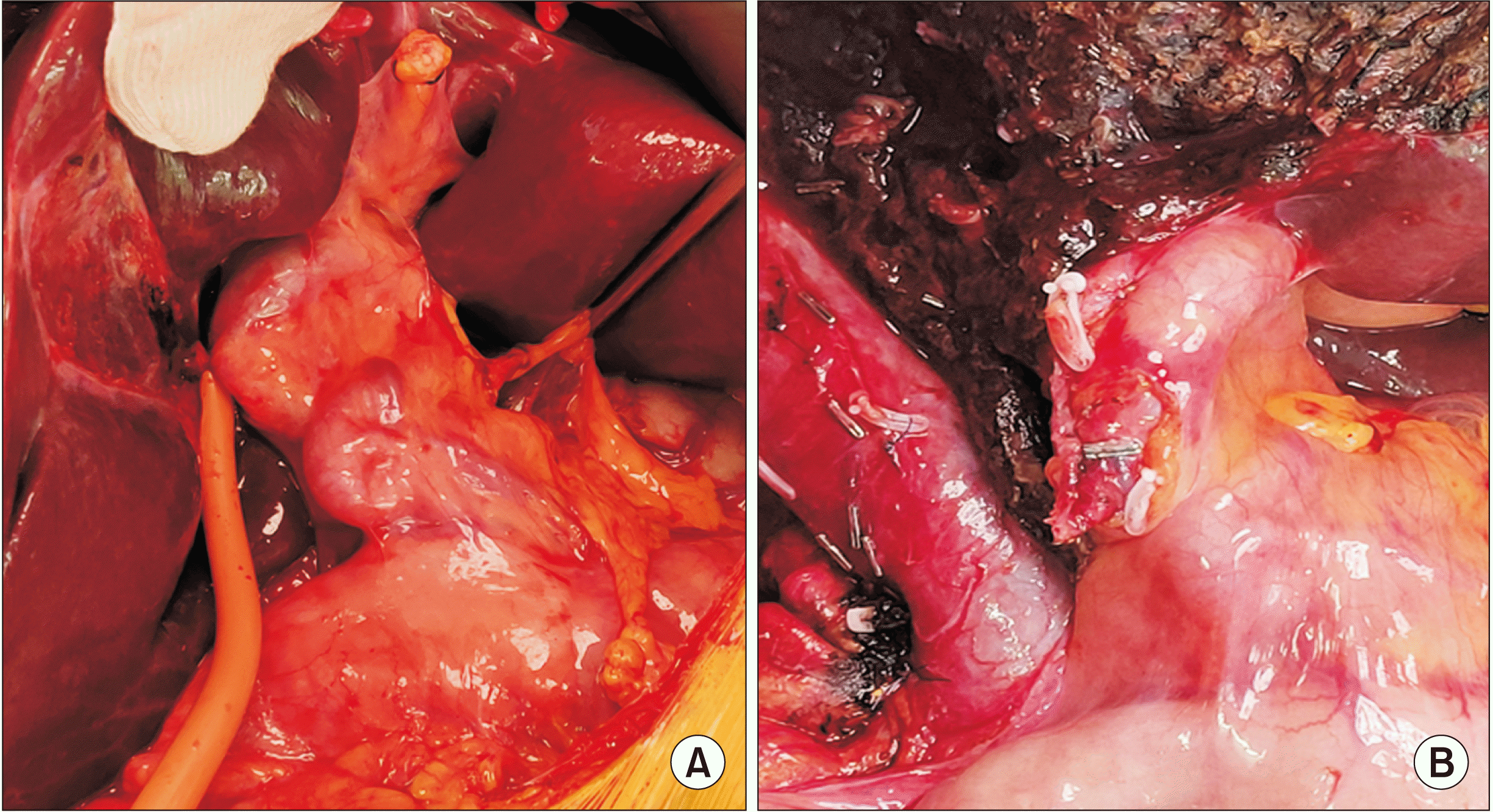
Fig. 3
Histopathological slides of hepatic angiomyolipoma. Low magnification view shows well delineated mass containing adipose tissue and irregular large blood vessels (A). High power view shows three components of angiomyolipoma: adipose tissue, blood vessels, and epithelioid to spindle cells (B). Immunohistochemistry shows HMB45 expression (C) and smooth muscle actin expression (D) of tumor cells.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download