Abstract
Backgrounds/Aims
Post-operative pancreatic fistulas (POPF) and fluid collections (POPFC) remain significant sources of morbidity and mortality after pancreatic resections. There remains a paucity of literature describing endoscopic ultrasound (EUS) guided drainage of POPFC using a Hot AXIOS™ lumen apposing metal stent (LAMS).
Methods
We conducted a retrospective study, encompassing all consecutive patients with POPFC managed using Hot AXIOS™ LAMS at our institution between January 2017 and December 2019. Primary outcome measures were technical and clinical success. Secondary outcome measures were adverse events and recurrence rates.
Results
Five patients underwent EUS guided drainage using Hot AXIOS™ LAMS during the study period. Mean age of patients was 67.8 ± 2.16 years. The majority (60.0%) of patients were males. Median duration of symptom onset after surgery was 9 days. All patients presented with abdominal pain. Median size of the collection measured on computed tomography was 91 mm. Median interval time between symptom onset and EUS drainage was 30 days. Two patients required percutaneous drainage prior to EUS guided drainage. Technical and clinical success were achieved for all patients. No adverse events were observed. Median duration of follow-up was 90 days. No recurrence of collection occurred during the follow-up period.
Infected post-operative pancreatic fistulas (POPF) and fluid collections (POPFC) are major sources of morbidity and mortality following pancreatic resections. The majority of collections that occur following distal pancreatectomies are due to a persistent leak from the pancreatic stump. The existing literature demonstrates that 30%–40% of patients may suffer from a clinically significant POPF leading to a collection following a distal pancreatectomy [1-3]. A recent systematic review and meta-analysis has shown that the incidence of POPF is higher after a central pancreatectomy than that after a distal pancreatectomy [4]. In those undergoing a distal pancreatectomy, predisposing factors for POPF include technique of pancreatic transection and stump closure, site of resection, and additional splenectomy [5-8]. POPFC has three grades based on clinical significance and the need for intervention. Grade A has been recently reassigned the term of biochemical leak as it does not affect clinical outcome of the patient. Grades B and C are clinically relevant that require further intervention [9]. Grade C is the most severe one. It may be fatal in 25% of patients [10]. The majority of POPFC are asymptomatic. Symptomatic collections may present with pain, fever, gastric outlet obstruction, fistulae, intra-abdominal sepsis, and multi-organ failure. Conservative management of patients with POPFC includes octreotide infusion, intravenous antibiotics, and total parenteral nutrition. Patients with Grade B and Grade C fistula require interventional therapy in the form of percutaneous or endoscopic drainage. Percutaneous drainage (PCD) is widely used because of its higher rate of technical success, widespread availability, safety, and low rate of adverse event or complications. PCD has its own limitations. It can leave an indwelling external catheter which may affect the overall quality of life. It also needs monitoring of drain output and frequent flushing. In addition, it requires trained health care professionals. Furthermore, serial upsizing of the drain may be required in addition to elective drain exchange [11-14]. Endoscopic management of pancreatic leak following pancreatic resection involves pancreatic sphincterotomy with or without trans-papillary stenting and endoscopic ultrasound (EUS) guided transmural drainage of POPFCs. Multiple case series have described the role of trans-papillary pancreatic duct stenting for POPFC as a safe and effective technique for management of POPF [15,16]. However, complications associated with endoscopic retrograde cholangiopancreatography (ERCP), especially post ERCP pancreatitis, lack of benefit in larger and infected collections, and collections not communicating with the pancreatic duct, are associated with a trans-papillary stenting.
There is significant evidence in the literature that supports the use of EUS guided transmural drainage of pancreatic fluid collections (PFCs) following pancreatitis [17]. EUS has revolutionised the management of PFCs. It has almost replaced other treatment modalities as the initial procedure of choice for drainage of PFCs following pancreatitis [18]. The role of EUS guided PFC drainage has evolved from plastic stents to fully covered self-expandable metal stents (fcSEMS) and most recently lumen apposing metal stents (LAMS), including Hot AXIOS™ stent (Boston Scientific, Marlborough, MA, USA), Nagi stent (Taewoong Medical Co., Goyang, Korea), and Niti-S SPAXUS stent (Taewoong Medical Co.) [19-24]. One advantage of the Hot AXIOS™ LAMS system is that it eliminates multiple steps during EUS guided drainage of PFC. Consequently, it is safer and more time-efficient than other LAMS and fcSEMS. Previous studies have evaluated the role of EUS guided drainage in POPFCs. However, the experience with Hot AXIOS™ for drainage of POPFCs has been limited [25,26]. In this service evaluation project, we report the safety and efficacy of LAMS Hot AXIOS™ for management of POPFCs based on our experience.
This was a service evaluation project involving retrospective analysis of routinely collected clinical data. Consecutive cases of POPF at our institution presenting between January 2017 and December 2019 were retrospectively identified from the database. Patients with POPFC grades B and C who underwent EUS guided drainage using Hot AXIOS™ LAMS were included in the analysis. EUS guided drainage was performed after multidisciplinary discussion between surgeons, interventional radiologists, and endoscopists. Symptomatic patients with well-defined walled off collections having close adherence to stomach or duodenum were chosen for EUS guided drainage. All patients underwent contrast enhanced computed tomography (CT) before drainage per our institute’s policy (Fig. 1–3). Informed consent was obtained from all patients prior to drainage. Patients less than 18 years of age, pregnant patients, patients with collections due to causes other than pancreatic resection, and patients with collections not amenable to EUS guided drainage were excluded from this study.
Data were collected using institutional electronic patient record and entered into a pre-designed excel spreadsheet. Demographic details including age, sex, nature of underlying disease and surgery, time interval between surgery and first symptom, time interval between onset of symptom and EUS drainage, imaging results (single vs. multiple collections, size of the largest collection), laboratory results (white blood cell count, C reactive protein at the time of admission when POPFC was first detected), and follow-up duration were recorded. Regarding EUS guided drainage, size of the collection, calibre of Hot AXIOS™ LAMS inserted, and LAMS indwelling time before removal were recorded. In addition, whether PCD was performed prior to EUS drainage and whether abdominal drain was removed before EUS drainage were also noted. Follow-up assessment was conducted either in the hospital’s outpatient clinic or by telephone.
EUS guided drainage using LAMS Hot AXIOS™ was performed by experienced endosonographers and team under propofol sedation or general anaesthesia. All procedures were carried out using a Pentax linear echo-endoscope (EG-3870-UTK; PENTAX House, Slough, United Kingdom). LAMS (Hot AXIOS™ stent and electrocautery-enhanced delivery system; Boston Scientific) used in this study were through-the-scope fcSEMS of 15 or 20 mm in diameter and 10 mm in length. After direct puncture of the wall of the collection using the electrocautery tip (without using a guidewire to assist in stent insertion), the delivery catheter was advanced into the fluid collection and the distal flange was deployed under EUS guidance (Fig. 4–7). The proximal flange was released under EUS or endoscopic view. A contrast enhanced CT of the abdomen and pelvis was performed for all patients before removing the LAMS (Fig. 8, 9). The LAMS was removed within 6 weeks for all patients following repeat cross-sectional imaging at 4–5 weeks (Fig. 10). It is our routine practice to subsequently replace the LAMS with two double pigtail plastic stents using the same tract.
Primary outcomes were technical and clinical success. Technical success was defined as successful placement of Hot AXIOS™ LAMS. Clinical success was defined as resolution of POPFC as confirmed on CT before removing Hot AXIOS™ LAMS.
A total of 434 patients underwent pancreatic and or splenic resections between January 2017 and December 2019. Twenty-three patients had POPFC and 18 (78.2%) patients had resolution of POPFC with conservative management or PCD. One patient required re-laparotomy and wash out. Five patients underwent EUS guided drainage using Hot AXIOS™ LAMS. Demographic details including underlying surgery details are summarized in Table 1. In the EUS drainage group, there were two patients who underwent distal pancreatectomy and splenectomy for neuroendocrine tumour of the pancreas, two who underwent splenectomy for B cell lymphoma and traumatic splenic rupture, respectively, and one who underwent subtotal pancreatectomy and splenectomy for pancreatic ductal adenocarcinoma.
Clinical details are summarised in Table 2. All patients presented with abdominal pain. In addition, two (40.0%) patients had clinical features of sepsis. All patients had raised C reactive protein levels. All but one patient had raised total leukocyte count at the time of initial presentation with POPFC. The size of the smallest collection was 83 mm (patient 2) and the largest collection had a size of 135 mm (patient 5). Multiple communicating collections were observed in 3 (60.0%) patients.
The median interval between the onset of symptom due to POPFC and EUS drainage was 30 days. In one patient, it was performed at 3 weeks after the onset of symptoms. In all patients, the abdominal drain inserted intraoperatively had been removed before the onset of symptoms. Two of five patients underwent PCD before the EUS guided drainage. Two patients had LAMS of 15 mm in diameter inserted and the remainder had 20 mm in diameter. Technical success and clinical success rates were 100% in all patients. LAMS was removed after successful resolution of the collection by 6 weeks in all patients. No major or minor adverse events were encountered. The median follow-up duration was 90 days (range, 30–364 days). No recurrence was observed in the study population in the follow-up period. Microbiological profiles of fluids aspirated from collections during EUS guided drainage are summarised in Table 3. Fluids from patient 1 and 5 had growth of commensal flora while patients 2, 3, and 4 showed growths of pathogenic microorganisms in addition to commensal flora. Patient-wise demographic details are summarised in Table 4. Three of our patients had a large hiatus hernia. If post-pancreatectomy collection extends superiorly, it may present a technical challenge. Thus, it is important to avoid deploying the LAMS within a hiatus pouch.
The Hot AXIOS™ stent system purports to offer a larger diameter stent, superior anchorage, ease of use, limited procedural time, and minimal complications. Our findings are concordant with the previously published case reports and series in this particular clinical context. Li et al. [25] have described the use of Hot AXIOS™ in a 59-year-old patient with pseudocyst at 4 months following distal pancreatectomy and splenectomy. A 15 × 10 mm stent was used for drainage which was removed 60 days later. No adverse event or recurrence was observed. Li et al. [25] removed the LAMS after 60 days while in our study we removed the LAMS by 6 weeks as there was evidence that removal of LAMS after 6 weeks could increase the risk of complications, especially bleeding. To date, Mudireddy et al. [26] have published the largest case series on the use of LAMS for management of post-surgical collections. However, the population in their multi-centric cohort was quite heterogeneous. Of 47 patients, 26 (55.3%) had fluid collections after pancreatic resections. The authors used both electrocautery enhanced (hot) LAMS and non-electrocautery enhanced LAMS. The authors used LAMS of 15 mm and 10 mm in size in 70% and 30% patients, respectively, while in our study, 15 mm and 20 mm LAMS were used in 40% and 60% of patients, respectively. Forty percent of our patients had attempted PCD prior to EUS guided drainage in the present study. However, in the study of Mudireddy et al. [26], 30.7% of 26 patients had PCD. The authors repeated the imaging after drainage at 1–8 weeks at the discretion of the endoscopist while we routinely performed a repeat imaging at 4–5 weeks after EUS drainage. The authors also described through scope dilatation of LAMS immediately after placement which was not a strategy utilised by our unit. Despite all these differences in protocols, they reported technical and clinical success rates of 100% and 96%, respectively.
Priyanka et al. [27] have described three cases of post-surgical PFCs successfully managed with EUS guided LAMS drainage. The first patient underwent Whipple procedure for pancreatic cancer and developed symptomatic PFC at five months after the surgery. An EUS guided drainage of the collection was performed using 10 mm LAMS. Unfortunately, the patient succumbed to the illness. The second patient had a pancreatic leak from the tail following neuroendocrine tumour surgery and underwent ERCP guided trans-papillary stenting of the pancreatic duct. However, the PFC persisted. It was drained using 15 mm calibre LAMS. The patient eventually died following a neurological event. The third patient presented with multi-organ injury following a road traffic accident and underwent splenectomy and hepatectomy. He developed a pancreatic leak one week later. He was initially managed with trans-papillary pancreatic duct stenting. Subsequently he presented with sepsis and a PFC. Following a failed PCD, EUS guided LAMS drainage of the collection was performed using 15 mm LAMS. The patient had an uneventful recovery. The stent was removed at eight weeks after the insertion. Technical and clinical success were achieved for all three patients in the series reported by Priyanka et al. [27]. Two of three patients underwent ERCP guided pancreatic stenting before EUS guided drainage. Unfortunately, two of the three patients died from events unrelated to the drainage [27].
However, none of the aforementioned series was focussed on challenges of EUS guided Hot AXIOS™ drainage of post-surgical pancreatic collections in comparison with drainage of pancreatitis related collections. As described in our results, POPFC are associated with a technical challenge of stent placement. These collections are often lying in a superior position in relation to the fundus of the stomach compared to collections seen following pancreatitis which are more commonly lying inferiorly. This poses a risk of deployment of the proximal flange of the stent in the esophagus, especially if patients have hiatus hernia as evident in three patients in our study. Another challenge in these collections is recurrent obstruction of the stent lumen by food debris as stents are often deployed immediately inferior to the gastroesophageal junction due to anatomical limitations. In addition, there is an increased potential risk of diaphragmatic injury while puncturing the collection again due to a close anatomical relationship with these collections.
Denzer et al. [28] have conducted a retrospective study on the role of EUS guided drainage using plastic stents for PFCs following pancreatic surgery. Twenty patients underwent 24 procedures and drainage was performed within a median duration of 30 days. Mean size of the collection was 72.5 mm and PCD was done in four patients before EUS guided drainage. Technical success rate was 100% and clinical success rate was 90%. Only one patient had a recurrence. This patient was subsequently referred for surgical management. Varadarajulu et al. [29] have published a case series on the management of EUS guided drainage of POPFC using double pigtail plastic stents with or without the use of naso-cystic drains. Out of 10 patients, successful drainage was achieved in nine patients. Only one patient had to undergo surgery for persistent symptoms. One patient in this series had stent migration. No other complications were observed. Three out of the ten patients had positive bacterial or fungal culture from aspirated fluid. Overall, authors concluded that EUS guided drainage was a safe and effective modality for managing POPFC.
Prior to introduction of EUS in the management of POPFC, PCD with or without trans-papillary pancreatic duct drainage was the modality of choice for the management of POPFC associated with pancreatic fistulas following surgery. Few studies have compared EUS with PCD for the management of POPFC. Kwon et al. [30] have performed a retrospective study comparing outcomes of POPFC management using percutaneous (PD) versus endosonographic approach (EUSD). Clinical success was achieved in 79% of patients in the PD group compared to 100% in the EUSD group. Three patients in the PD group had recurrence following drain removal. The recurrence was later managed successfully with EUSD. Futagawa et al. [31] have compared the use of EUS guided trans-gastric drainage (EUS-GD) of POPFC versus PCD in 33 patients retrospectively. Twenty-one (63.6%) patients underwent PCD and 12 (36.4%) patients underwent EUS-GD. Technical success was achieved for 92% of patients in the EUS-GD group compared with 100% in the PCD group. There were no statistically significant differences in outcome measures between the two groups. A recent systematic review and meta-analyses by Mohan et al. [32] has confirmed findings of these isolated studies and inferred that in terms of clinical outcome, EUS guided drainage is a safe, effective, and preferable modality for the management of POPFC. However, regarding adverse events and technical success rate, EUS guided drainage is similar to PCD. Results of our retrospective series are consistent with published literature, further strengthening the evidence supporting minimally invasive management of POPFC using LAMS device such as Hot AXIOS™.
Our data also highlight some important points in the management of POPFC. Technical and clinical success were achieved in all patients. No major or minor adverse events were observed even after a long-term follow-up (364 days). All patients had successful resolution of intra-abdominal collections and retrieval of the stent by 6 weeks. Our experience demonstrated that EUS guided drainage using Hot AXIOS™ LAMS could be performed as early as three weeks from the detection of POPFC. This is not always possible as the development of a wall around the POPFC usually takes 4–6 weeks. The first line treatment of POPFC is often PCD under intervention radiology guidance.
Limitations of our study include its restriction to a single centre, the retrospective design, and the limited number of patients. It was impossible to have a comparative arm with this volume of patients. The current cost of Hot AXIOS™ LAMS might also be another limiting factor for consideration in the management of POPFC. However, compared to PCD, repeat surgery, and complications associated with trans-papillary drainage, EUS guided drainage using Hot AXIOS™ LAMS appears to be more economical. We conclude that EUS guided drainage of POPFC using Hot AXIOS™ LAMS is a safe and effective technique. Further large-scale prospective studies are needed determine the role of this technique in wider practice management of POPFC.
REFERENCES
1. Jayaraman S, Gonen M, Brennan MF, D'Angelica MI, DeMatteo RP, Fong Y, Jarnagin WR, et al. 2010; Laparoscopic distal pancreatectomy: evolution of a technique at a single institution. J Am Coll Surg. 211:503–509. DOI: 10.1016/j.jamcollsurg.2010.06.010. PMID: 20868976.

2. Ferrone CR, Warshaw AL, Rattner DW, Berger D, Zheng H, Rawal B, et al. 2008; Pancreatic fistula rates after 462 distal pancreatectomies: staplers do not decrease fistula rates. J Gastrointest Surg. 12:1691–1697. discussion 1697–1698. DOI: 10.1007/s11605-008-0636-2. PMID: 18704597. PMCID: PMC3806097.

3. Kleeff J, Diener MK, Z'graggen K, Hinz U, Wagner M, Bachmann J, et al. 2007; Distal pancreatectomy: risk factors for surgical failure in 302 consecutive cases. Ann Surg. 245:573–582. DOI: 10.1097/01.sla.0000251438.43135.fb. PMID: 17414606. PMCID: PMC1877036.
4. Iacono C, Verlato G, Ruzzenente A, Campagnaro T, Bacchelli C, Valdegamberi A, et al. 2013; Systematic review of central pancreatectomy and meta-analysis of central versus distal pancreatectomy. Br J Surg. 100:873–885. DOI: 10.1002/bjs.9136. PMID: 23640664.

5. Benoist S, Dugué L, Sauvanet A, Valverde A, Mauvais F, Paye F, et al. 1999; Is there a role of preservation of the spleen in distal pancreatectomy? J Am Coll Surg. 188:255–260. DOI: 10.1016/S1072-7515(98)00299-3. PMID: 10065814.

6. Shankar S, Theis B, Russell RC. 1990; Management of the stump of the pancreas after distal pancreatic resection. Br J Surg. 77:541–544. DOI: 10.1002/bjs.1800770525. PMID: 2354339.

7. Sheehan MK, Beck K, Creech S, Pickleman J, Aranha GV. 2002; Distal pancreatectomy: does the method of closure influence fistula formation? Am Surg. 68:264–267. discussion 267–268. PMID: 11893105.
8. Suzuki Y, Fujino Y, Tanioka Y, Hori Y, Ueda T, Takeyama Y, et al. 1999; Randomized clinical trial of ultrasonic dissector or conventional division in distal pancreatectomy for non-fibrotic pancreas. Br J Surg. 86:608–611. DOI: 10.1046/j.1365-2168.1999.01120.x. PMID: 10361178.

9. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. 2017; The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 161:584–591. DOI: 10.1016/j.surg.2016.11.014. PMID: 28040257.
10. Pedrazzoli S. 2017; Pancreatoduodenectomy (PD) and postoperative pancreatic fistula (POPF): a systematic review and analysis of the POPF-related mortality rate in 60,739 patients retrieved from the English literature published between 1990 and 2015. Medicine (Baltimore). 96:e6858. DOI: 10.1097/MD.0000000000006858. PMID: 28489778. PMCID: PMC5428612.
11. Men S, Akhan O, Köroğlu M. 2002; Percutaneous drainage of abdominal abcess. Eur J Radiol. 43:204–218. DOI: 10.1016/S0720-048X(02)00156-0. PMID: 12204403.

12. Freeny PC, Hauptmann E, Althaus SJ, Traverso LW, Sinanan M. 1998; Percutaneous CT-guided catheter drainage of infected acute necrotizing pancreatitis: techniques and results. AJR Am J Roentgenol. 170:969–975. DOI: 10.2214/ajr.170.4.9530046. PMID: 9530046.

13. Matthews JB. 2011; Prevention, evaluation, and treatment of leaks after pancreatic surgery. J Gastrointest Surg. 15:1327–1328. DOI: 10.1007/s11605-011-1516-8. PMID: 21499953.

14. Vin Y, Sima CS, Getrajdman GI, Brown KT, Covey A, Brennan MF, et al. 2008; Management and outcomes of postpancreatectomy fistula, leak, and abscess: results of 908 patients resected at a single institution between 2000 and 2005. J Am Coll Surg. 207:490–498. DOI: 10.1016/j.jamcollsurg.2008.05.003. PMID: 18926450.

15. Goasguen N, Bourrier A, Ponsot P, Bastien L, Lesurtel M, Prat F, et al. 2009; Endoscopic management of pancreatic fistula after distal pancreatectomy and enucleation. Am J Surg. 197:715–720. DOI: 10.1016/j.amjsurg.2008.03.005. PMID: 18789426.

16. Reddymasu SC, Pakseresht K, Moloney B, Alsop B, Oropezia-Vail M, Olyaee M. 2013; Incidence of pancreatic fistula after distal pancreatectomy and efficacy of endoscopic therapy for its management: results from a tertiary care center. Case Rep Gastroenterol. 7:332–339. DOI: 10.1159/000354136. PMID: 24019766. PMCID: PMC3764947.

17. Puri R, Thandassery RB, Alfadda AA, Kaabi SA. 2015; Endoscopic ultrasound guided drainage of pancreatic fluid collections: assessment of the procedure, technical details and review of the literature. World J Gastrointest Endosc. 7:354–363. DOI: 10.4253/wjge.v7.i4.354. PMID: 25901214. PMCID: PMC4400624.

18. Arvanitakis M, Dumonceau JM, Albert J, Badaoui A, Bali MA, Barthet M, et al. 2018; Endoscopic management of acute necrotizing pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) evidence-based multidisciplinary guidelines. Endoscopy. 50:524–546. DOI: 10.1055/a-0588-5365. PMID: 29631305.

19. Bang JY, Varadarajulu S. 2013; Metal versus plastic stent for transmural drainage of pancreatic fluid collections. Clin Endosc. 46:500–502. DOI: 10.5946/ce.2013.46.5.500. PMID: 24143311. PMCID: PMC3797934.

20. Sharaiha RZ, DeFilippis EM, Kedia P, Gaidhane M, Boumitri C, Lim HW, et al. 2015; Metal versus plastic for pancreatic pseudocyst drainage: clinical outcomes and success. Gastrointest Endosc. 82:822–827. DOI: 10.1016/j.gie.2015.02.035. PMID: 25936453.

21. Penn DE, Draganov PV, Wagh MS, Forsmark CE, Gupte AR, Chauhan SS. 2012; Prospective evaluation of the use of fully covered self-expanding metal stents for EUS-guided transmural drainage of pancreatic pseudocysts. Gastrointest Endosc. 76:679–684. DOI: 10.1016/j.gie.2012.04.457. PMID: 22732874.

22. Siddiqui AA, Adler DG, Nieto J, Shah JN, Binmoeller KF, Kane S, et al. 2016; EUS-guided drainage of peripancreatic fluid collections and necrosis by using a novel lumen-apposing stent: a large retrospective, multicenter U.S. experience (with videos). Gastrointest Endosc. 83:699–707. DOI: 10.1016/j.gie.2015.10.020. PMID: 26515956.

23. Siddiqui AA, Kowalski TE, Loren DE, Khalid A, Soomro A, Mazhar SM, et al. 2017; Fully covered self-expanding metal stents versus lumen-apposing fully covered self-expanding metal stent versus plastic stents for endoscopic drainage of pancreatic walled-off necrosis: clinical outcomes and success. Gastrointest Endosc. 85:758–765. DOI: 10.1016/j.gie.2016.08.014. PMID: 27566053.

24. Rinninella E, Kunda R, Dollhopf M, Sanchez-Yague A, Will U, Tarantino I, et al. 2015; EUS-guided drainage of pancreatic fluid collections using a novel lumen-apposing metal stent on an electrocautery-enhanced delivery system: a large retrospective study (with video). Gastrointest Endosc. 82:1039–1046. DOI: 10.1016/j.gie.2015.04.006. PMID: 26014960.

25. Li L, Cristofaro S, Qu C, Liang S, Li X, Cai Q. 2018; EUS-guided drainage of pancreatic fluid collection with a Hot AXIOS stent in a patient with pancreatitis following distal pancreatectomy (with video). Endosc Ultrasound. 7:347–348. DOI: 10.4103/eus.eus_55_17. PMID: 29848832. PMCID: PMC6199910.

26. Mudireddy PR, Sethi A, Siddiqui AA, Adler DG, Nieto J, Khara H, et al. 2018; EUS-guided drainage of postsurgical fluid collections using lumen-apposing metal stents: a multicenter study. Gastrointest Endosc. 87:1256–1262. DOI: 10.1016/j.gie.2017.08.011. PMID: 28843581.

27. Priyanka P, Hsueh W, Nasr J. 2019; Use of Lumen-apposing stents for the treatment of postsurgical fluid collections: a case series and a review of literature. Case Rep Gastrointest Med. 2019:7656950. DOI: 10.1155/2019/7656950. PMID: 30809400. PMCID: PMC6364124.

28. Denzer UW, Sioulas AD, Abdulkarim M, Groth S, Rösch T, Busch P, et al. 2016; Endoscopic ultrasound-guided drainage of abdominal fluid collections after pancreatic surgery: efficacy and long-term follow-up. Z Gastroenterol. 54:1047–1053. DOI: 10.1055/s-0042-112032. PMID: 27612217.

29. Varadarajulu S, Trevino JM, Christein JD. 2009; EUS for the management of peripancreatic fluid collections after distal pancreatectomy. Gastrointest Endosc. 70:1260–1265. DOI: 10.1016/j.gie.2009.06.007. PMID: 19660749.

30. Kwon YM, Gerdes H, Schattner MA, Brown KT, Covey AM, Getrajdman GI, et al. 2013; Management of peripancreatic fluid collections following partial pancreatectomy: a comparison of percutaneous versus EUS-guided drainage. Surg Endosc. 27:2422–2427. DOI: 10.1007/s00464-012-2752-z. PMID: 23361258.

31. Futagawa Y, Imazu H, Mori N, Kanazawa K, Chiba M, Furukawa K, et al. 2017; The effectiveness and feasibility of endoscopic ultrasound-guided transgastric drainage of postoperative fluid collections early after pancreatic surgery. Surg Laparosc Endosc Percutan Tech. 27:267–272. DOI: 10.1097/SLE.0000000000000413. PMID: 28520649.

32. Mohan BP, Shakhatreh M, Dugyala S, Geedigunta V, Gadalay A, Pahal P, et al. 2019; EUS versus percutaneous management of postoperative pancreatic fluid collection: a systematic review and meta-analysis. Endosc Ultrasound. 8:298–309. DOI: 10.4103/eus.eus_18_19. PMID: 31249160. PMCID: PMC6791105.

Fig. 1
Pancreatic fluid collection at the resection site extending up to the under surface of the greater curvature of the stomach. Contrast enhanced computed tomography of abdomen (pre-endoscopic ultrasound drainage).
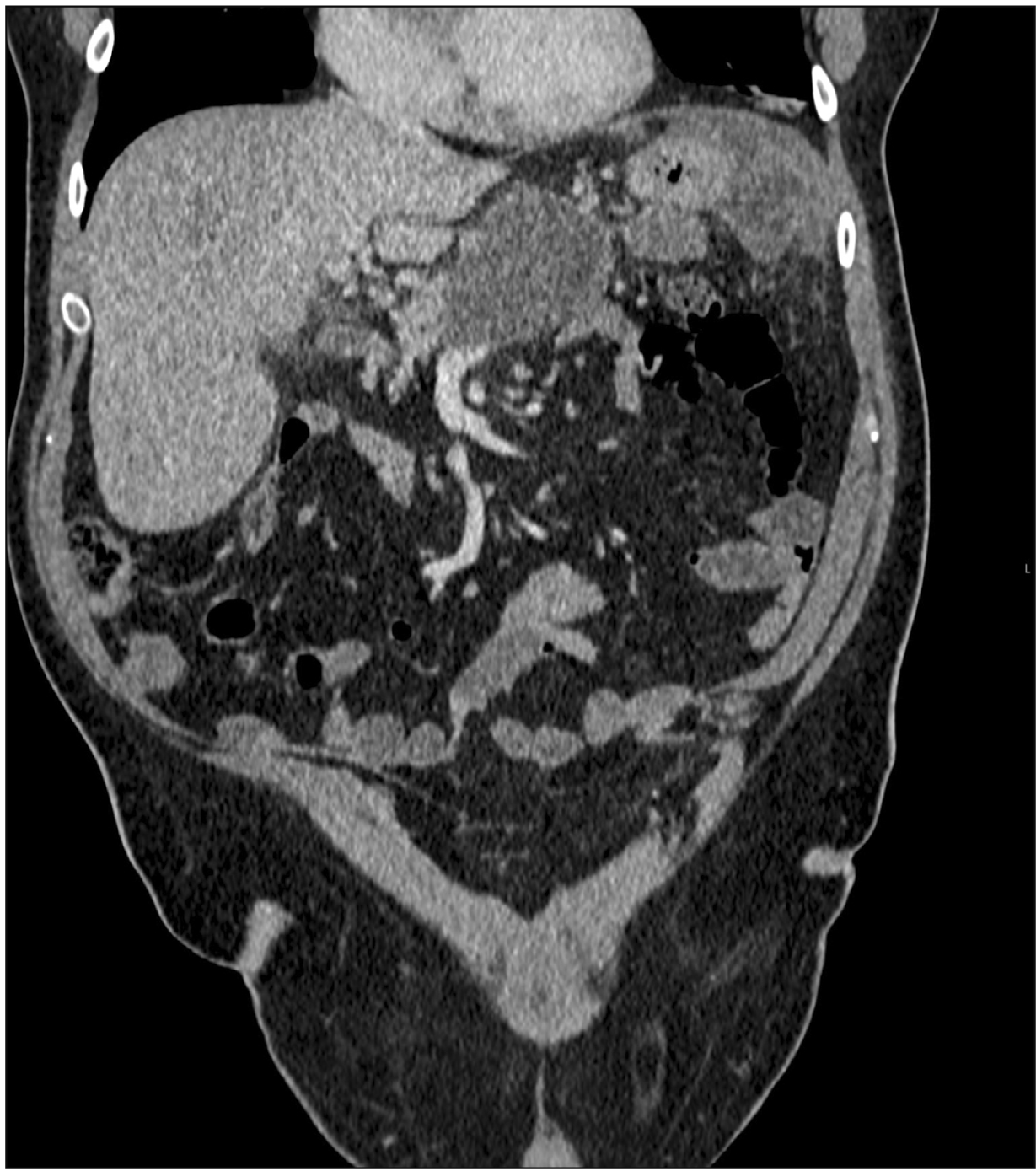
Fig. 2
Organised pancreatic fluid collection at the surgical bed with multiple locules of air (post percutaneous drainage). An incidental simple cyst of liver and a left tissue breast implant can also be seen. Contrast enhanced computed tomography of abdomen (pre-endoscopic ultrasound drainage).
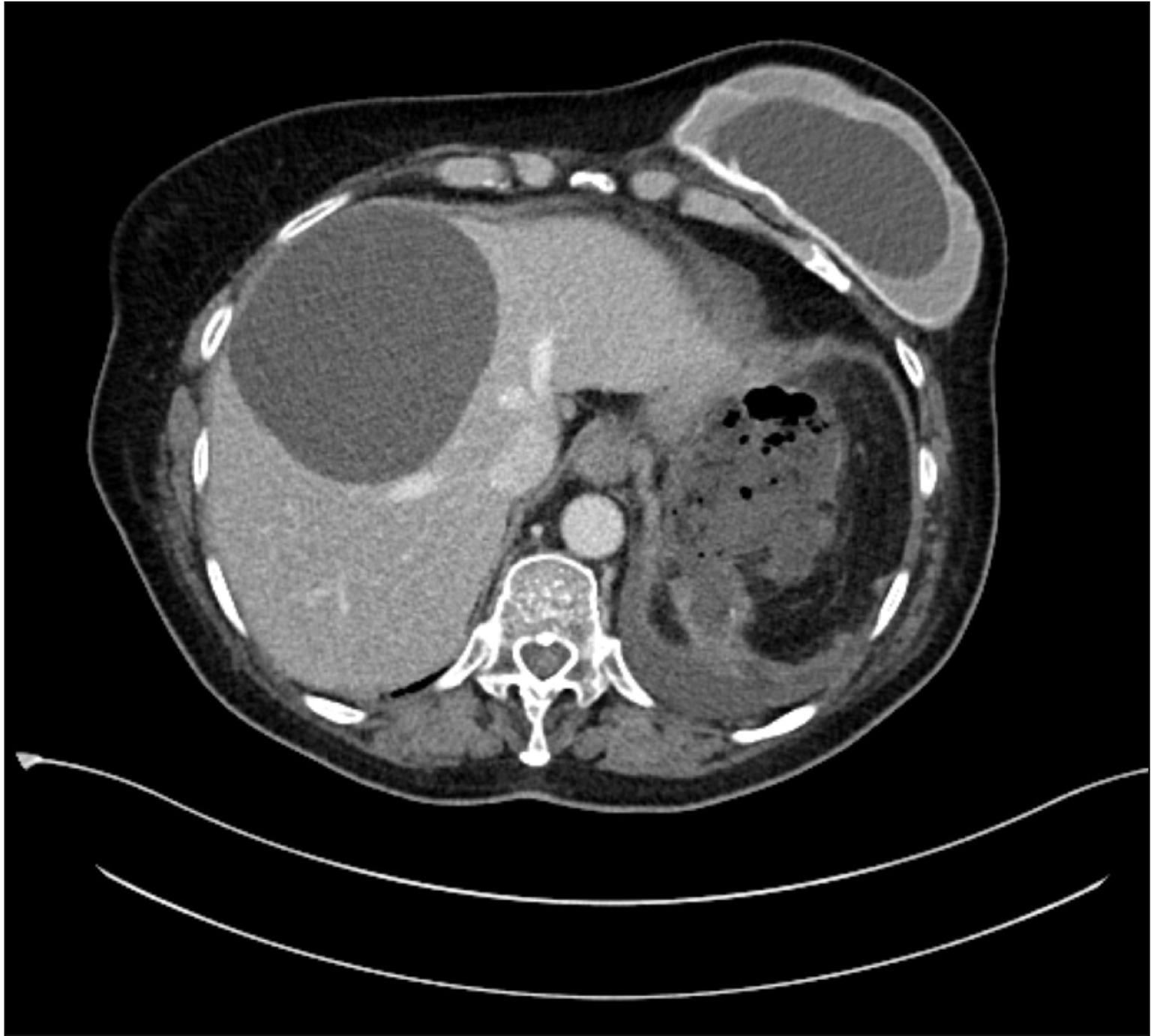
Fig. 3
Pancreatic fluid collection between the tail of the pancreas and the remnant spleen. Contrast enhanced computed tomography of abdomen (pre-endoscopic ultrasound drainage).
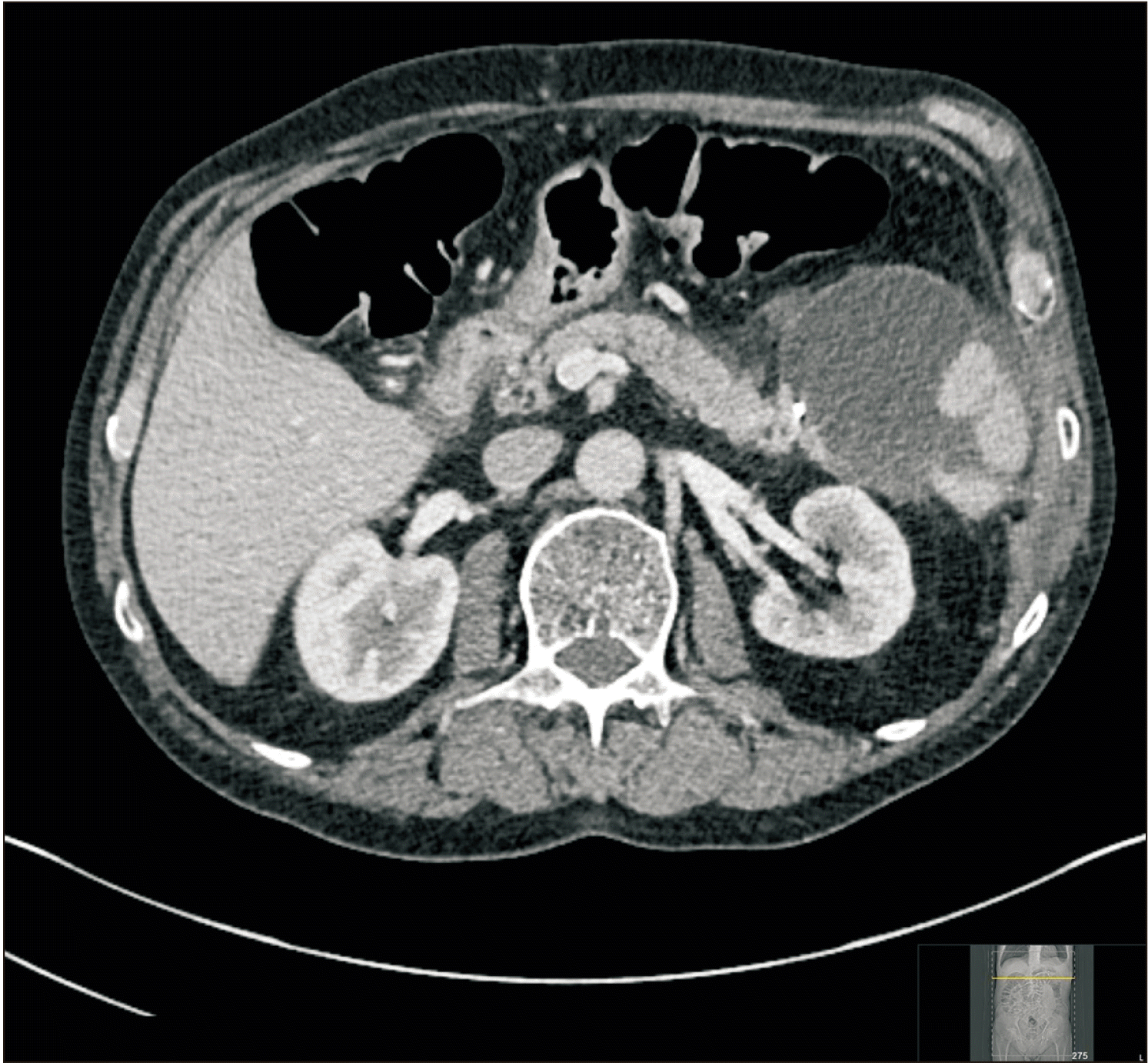
Fig. 4
Large pancreatic fluid collection of 6 cm in size in the visualized plane with echogenic component. Endoscopic ultrasound images.
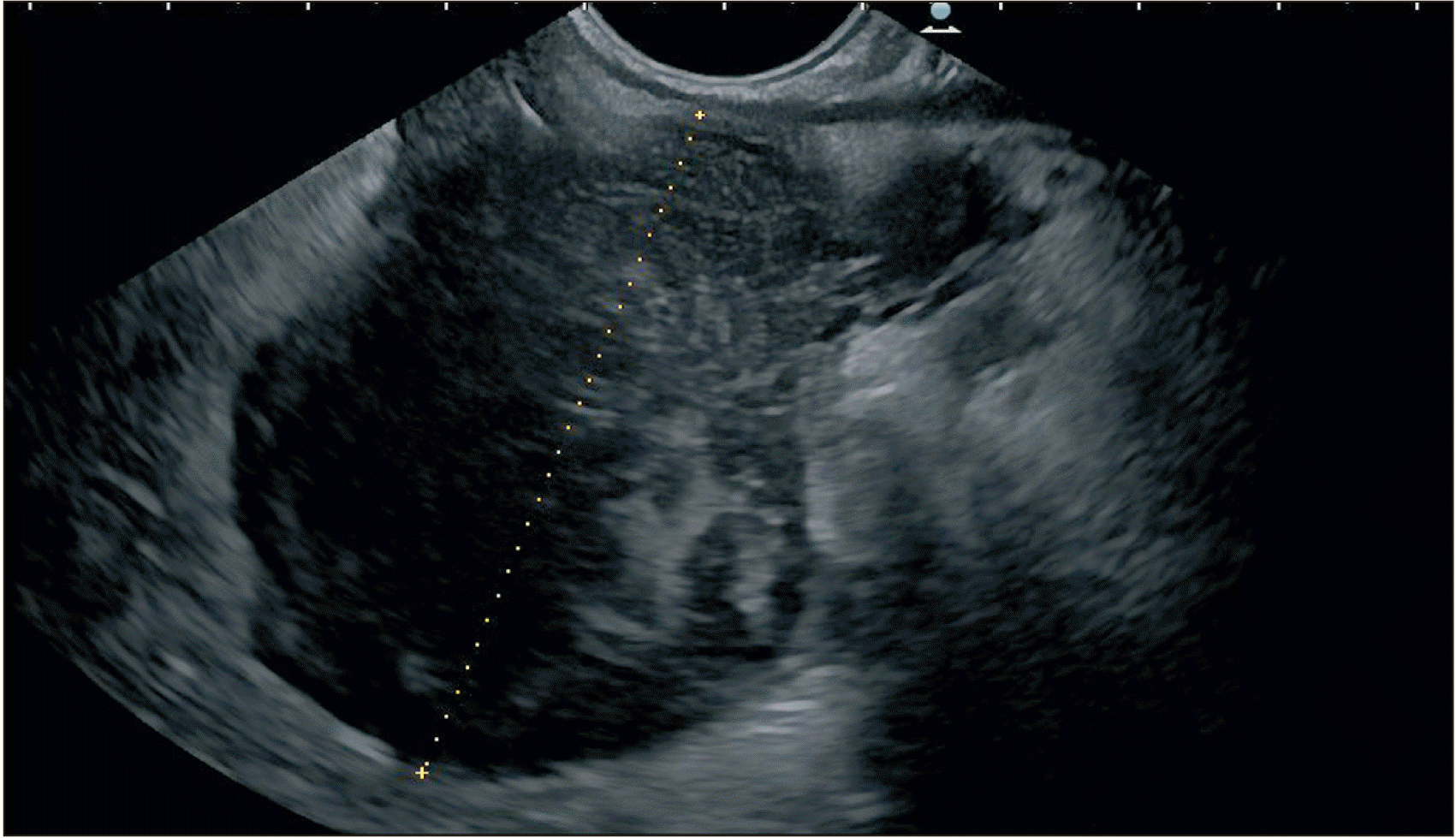
Fig. 5
A well-defined thick-walled collection with echogenic fluid consistent with pus. Endoscopic ultrasound images.
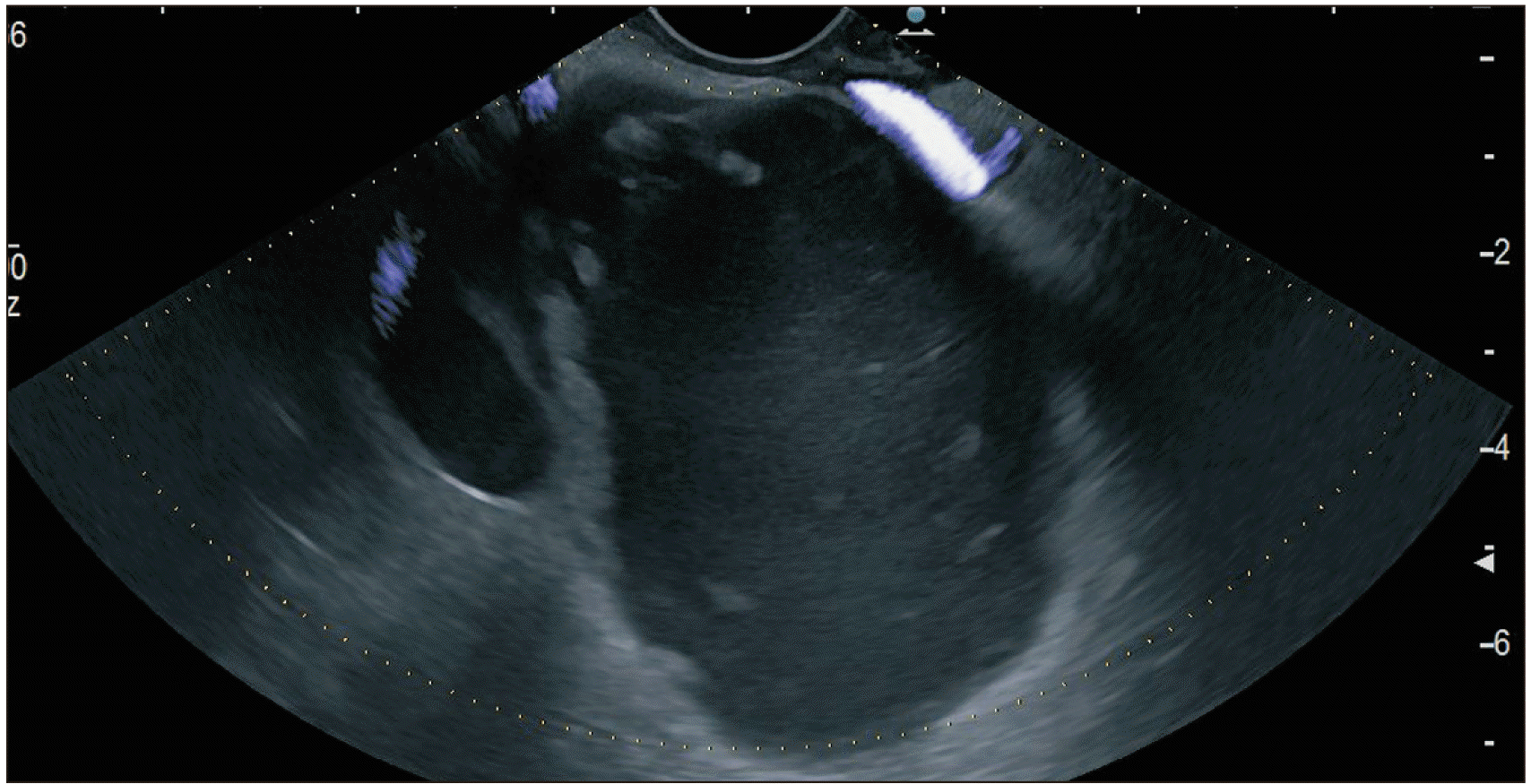
Fig. 6
Hot AXIOS™ stent with a delivery catheter and a deployed distal flange within the collection. Endoscopic ultrasound images.
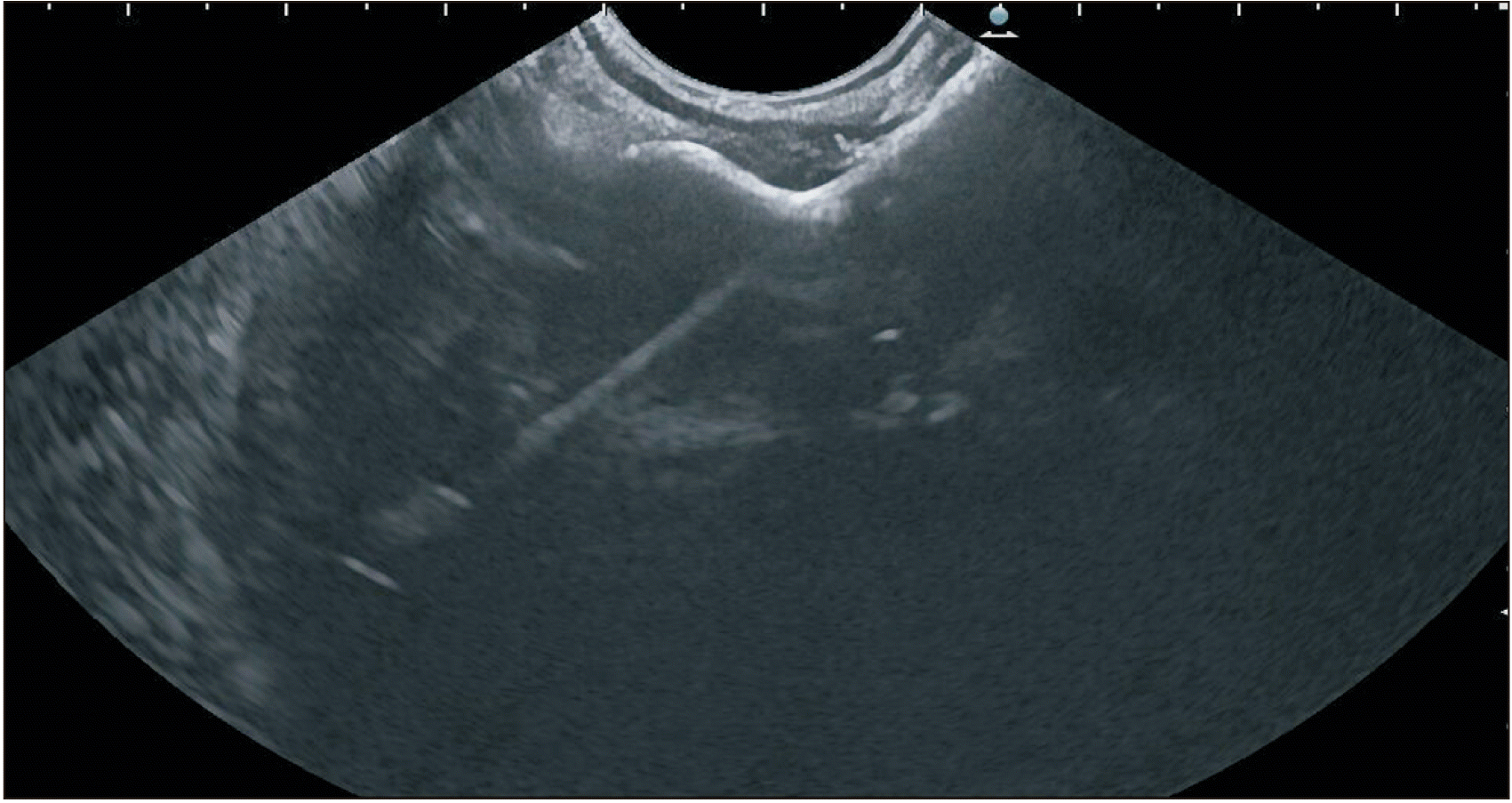
Fig. 7
Hot AXIOS™ stent with a delivery catheter and a deployed distal flange within the collection. Endoscopic ultrasound images.
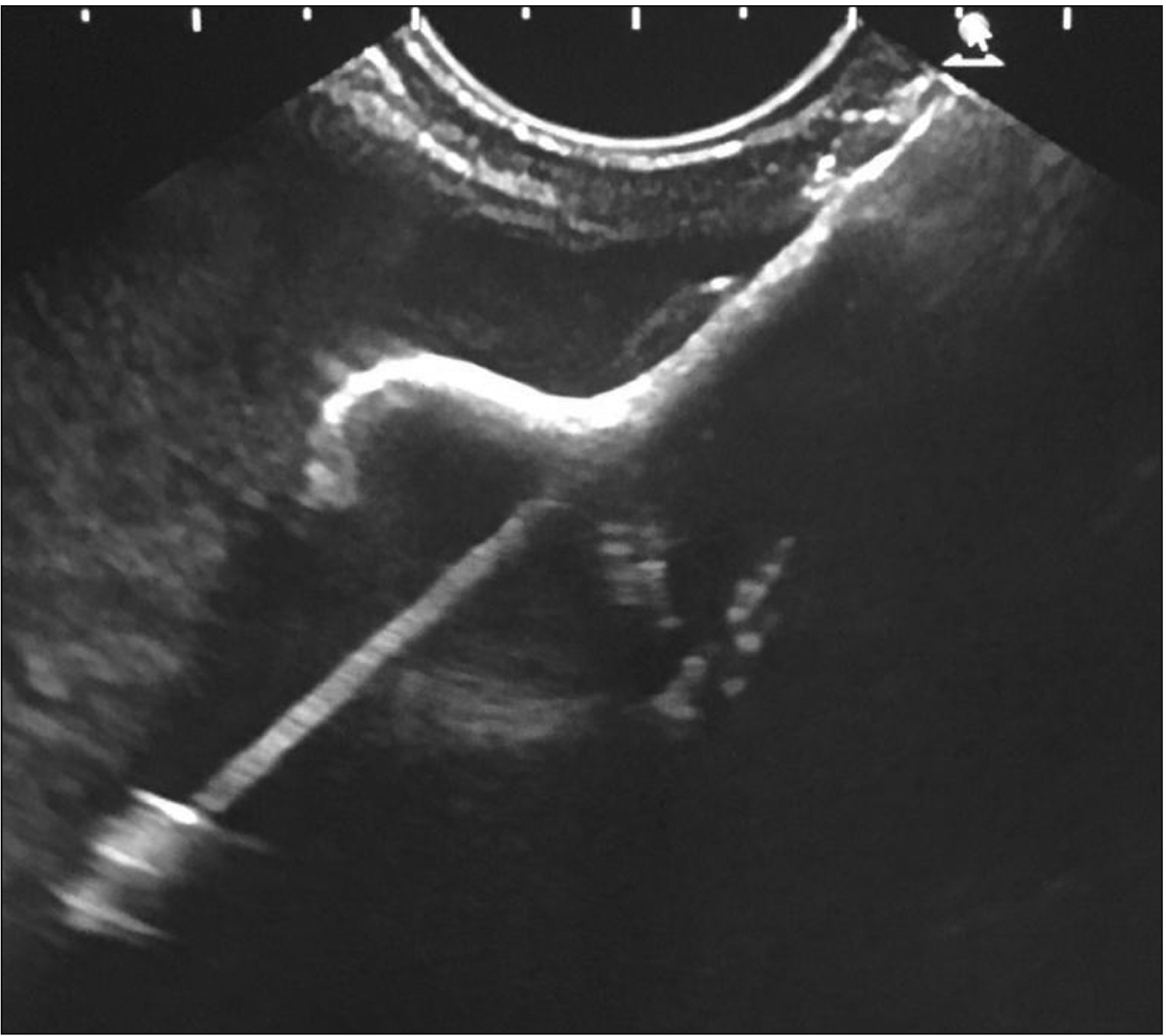
Fig. 8
Contrast enhanced computed tomography abdomen demonstrating marked reduction in the size of collection post drainage with lumen apposing metal stent in situ. Contrast enhanced computed tomography of abdomen (post-endoscopic ultrasound drainage and pre-removal of AXIOS).
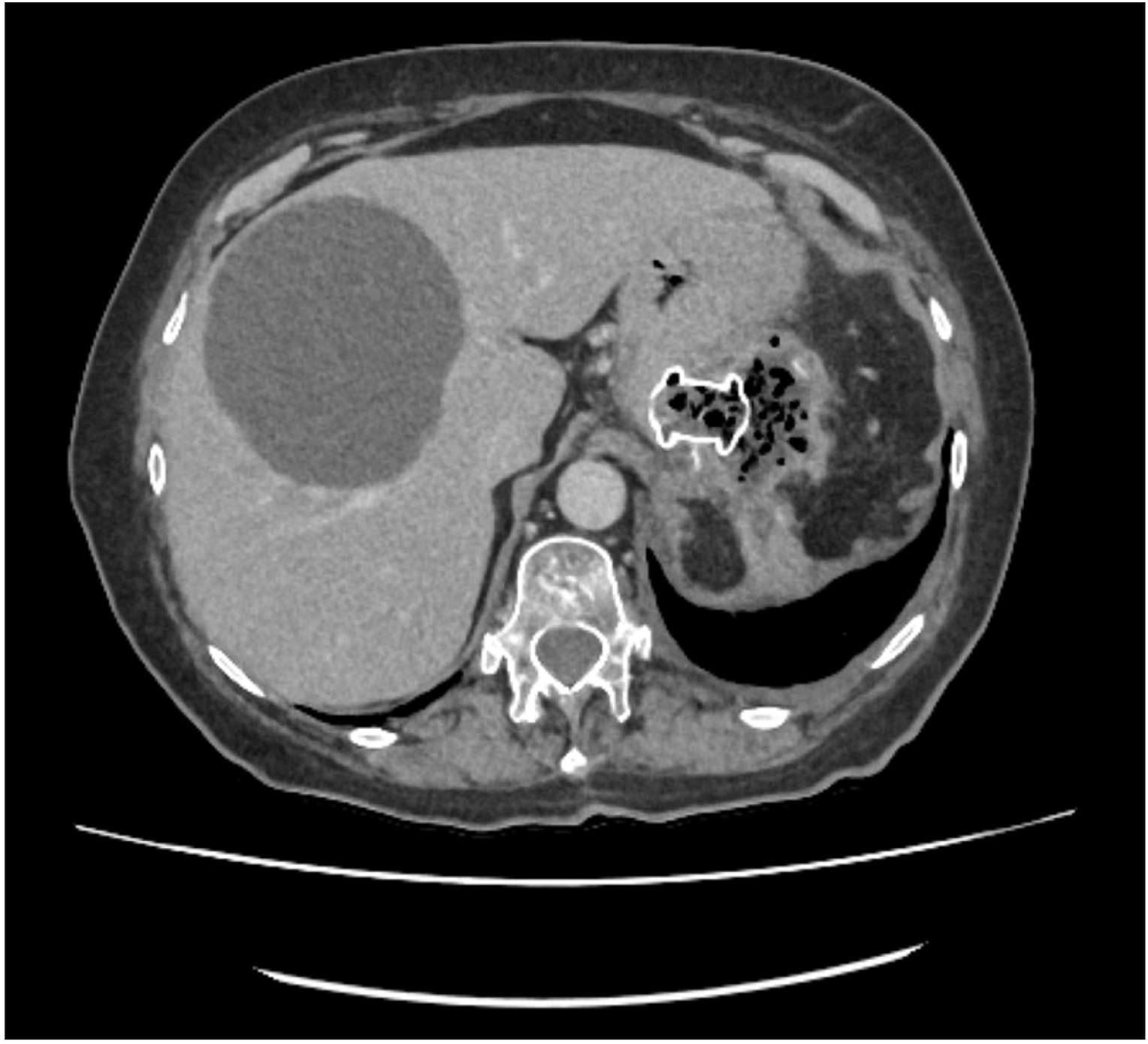
Fig. 9
Contrast enhanced computed tomography abdomen demonstrating complete resolution of the collection with lumen apposing metal stent in situ. Contrast enhanced computed tomography of abdomen (post-endoscopic ultrasound drainage and pre-removal of AXIOS).
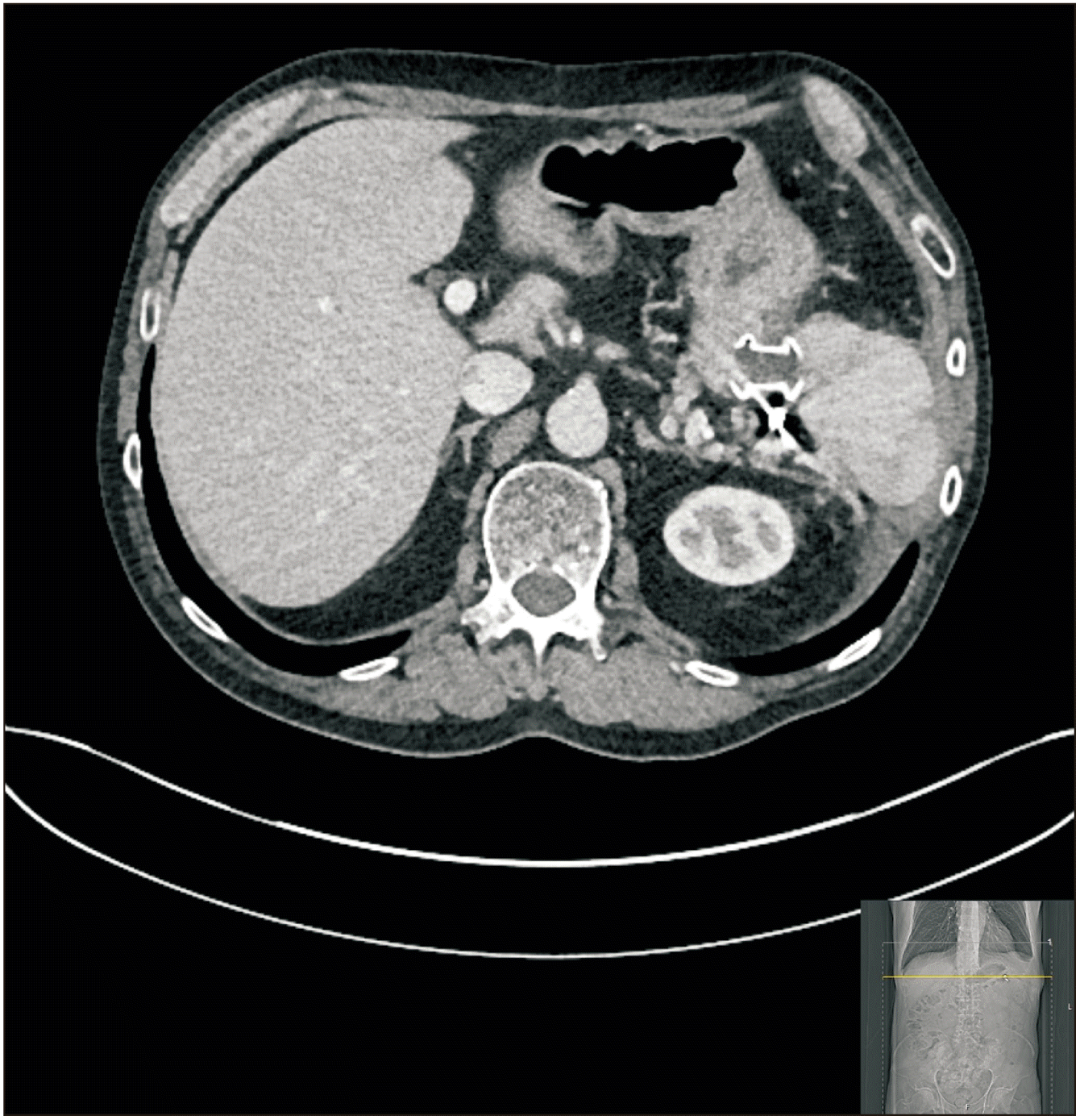
Table 1
Baseline demographic details and characteristics of five POPFC patients
Table 2
Clinico-radiological profile and outcome measures of five POPFC patients
Table 3
Microbiological profiles of drained collections of five POPFC patients
Table 4
Patient-wise demographic, clinical, and drainage details of five POPFC patients




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download