Abstract
Supported by the expanding indications for neoadjuvant therapy (NAT) for advanced pancreatic cancer (PC), the concept of resectability has evolved from being mostly based on the anatomical tumor extent to considering the biological and conditional factors relevant to prognosis. Therefore, it is more reasonable to define the “criteria for surgical resection” instead of using the “(technical) resectability criteria.” NAT has been used in resectable PCs (RPC) with a high risk of early systemic recurrence, as predicted by various biological or anatomical markers. Moreover, the indications for NAT followed by conversion surgery or adjuvant surgery for borderline resectable or locally advanced PC (LAPC) are gradually expanding. Therefore, it is important to define the RPC group that will benefit from NAT and the LAPC group that will benefit from post-NAT surgery. At diagnosis, population-based approaches, such as prognostic stratification and staging systems and personalized outcome-based approaches using prognostic prediction models can be used to determine the criteria for treatment options. Standardized indications for conversion surgery are needed for patients who are initially treated with NAT. In addition to imaging-based morphological criteria, biological criteria, including CA19-9, and various metabolic criteria can be used to establish predicted outcome-based criteria. Multicenter collaboration is required to develop a large database with standardized data collection for various biomarkers and response data after NAT to establish more accurate outcome prediction models to define the new resectability criteria.
Go to : 
Thus far, the resectability of solid tumors has been defined on the basis of safe and radical resection potential. Early and late morbidity, including surgical mortality and improved survival rates, are the most important factors in making rational decisions to perform cancer surgery. Pancreatic surgery is well-known for its high morbidity and mortality rates, and pancreatic ductal adenocarcinoma (PDAC) is a malignant tumor with the lowest resection rate among all solid tumors. Anatomical complexity, high surgical risk, and high systemic disease presentation are factors that make it difficult to determine resectability. Therefore, PDAC has been regarded as a systemic disease, and multimodal treatment has been emphasized [1,2].
With the advent and spread of neoadjuvant therapy (NAT) for advanced pancreatic cancer (PC), the concept of borderline resection potential has emerged as an indication for NAT instead of upfront surgery [3]. The conventional resectability criteria are mostly based on the anatomical tumor extent. However, biological factors relevant to prognosis are becoming increasingly important as many researchers have shown that biological tumor properties are related to the natural course of the tumor [4]. In some solid tumors, a personalized approach using various prognostic predictive models is becoming a reality. Therefore, in the multimodal setting for PC, the “criteria for surgical resection” instead of the “(technical) resectability criteria” seem more reasonable, turning the question of “Can it be surgically safely and radically removed?” into “Is surgery the best treatment option at this point?”
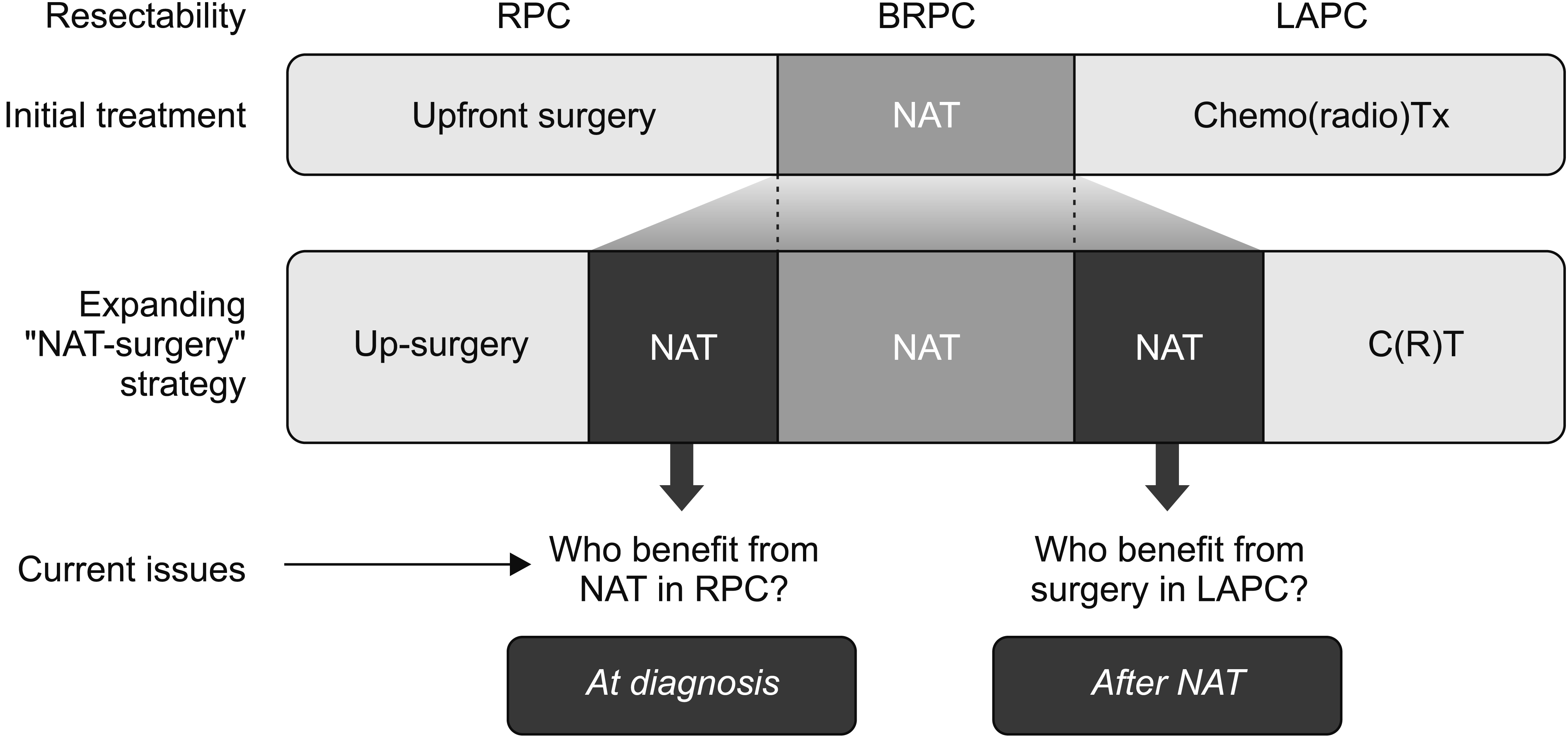
Upfront surgery is considered the standard treatment for resectable PC (RPC), and the best postoperative adjuvant therapy is still being studied [5,6]. Preoperative chemotherapy with or without radiation therapy as NAT has recently become a routine treatment for borderline RPC (BRPC) [7]. For locally advanced PC (LAPC), chemotherapy with or without radiation therapy is the primary therapy, and conversion surgery or adjuvant surgery has been attempted in many centers with reasonable outcomes [8]. Therefore, in addition to determining the treatment option at diagnosis, additional decision-making is required for subsequent treatment if the first treatment is NAT or non-surgical treatment. As can be seen from the initial treatment methods according to the resectability category, the indications for NAT are gradually expanding [9]. In addition, NAT has been used in RPC with a high risk of early systemic recurrence as predicted by various biological or anatomical markers [1]. Therefore, defining the RPC group that will benefit from NAT and the LAPC group that will benefit from post-NAT surgery is currently a prominent topic (Fig. 1).
Go to : 
Several guidelines and institutions have proposed various biological criteria for NAT in potentially RPC. The expert consensus International Association of Pancreatology guidelines redefined RPC with a CA19-9 level of > 500 U/mL and lymph node metastases confirmed by biopsy or positron emission tomography (PET) as BRPC, and NAT was recommended [10]. The National Comprehensive Cancer Network (NCCN) guidelines recommend NAT for RPC with high-risk features, including imaging findings, very high CA19-9 levels, large primary tumors, large regional lymph nodes, excessive weight loss, or extreme pain [1]. Many of these features are associated with a high likelihood of recurrence, but most are not clearly objective. Moon et al. [11] proposed a CA19-9 level of 150 U/mL and a PET-standardized uptake value (SUV) max of 5.5 as cutoff values to define the high-risk group, for which upfront surgery is not indicated.
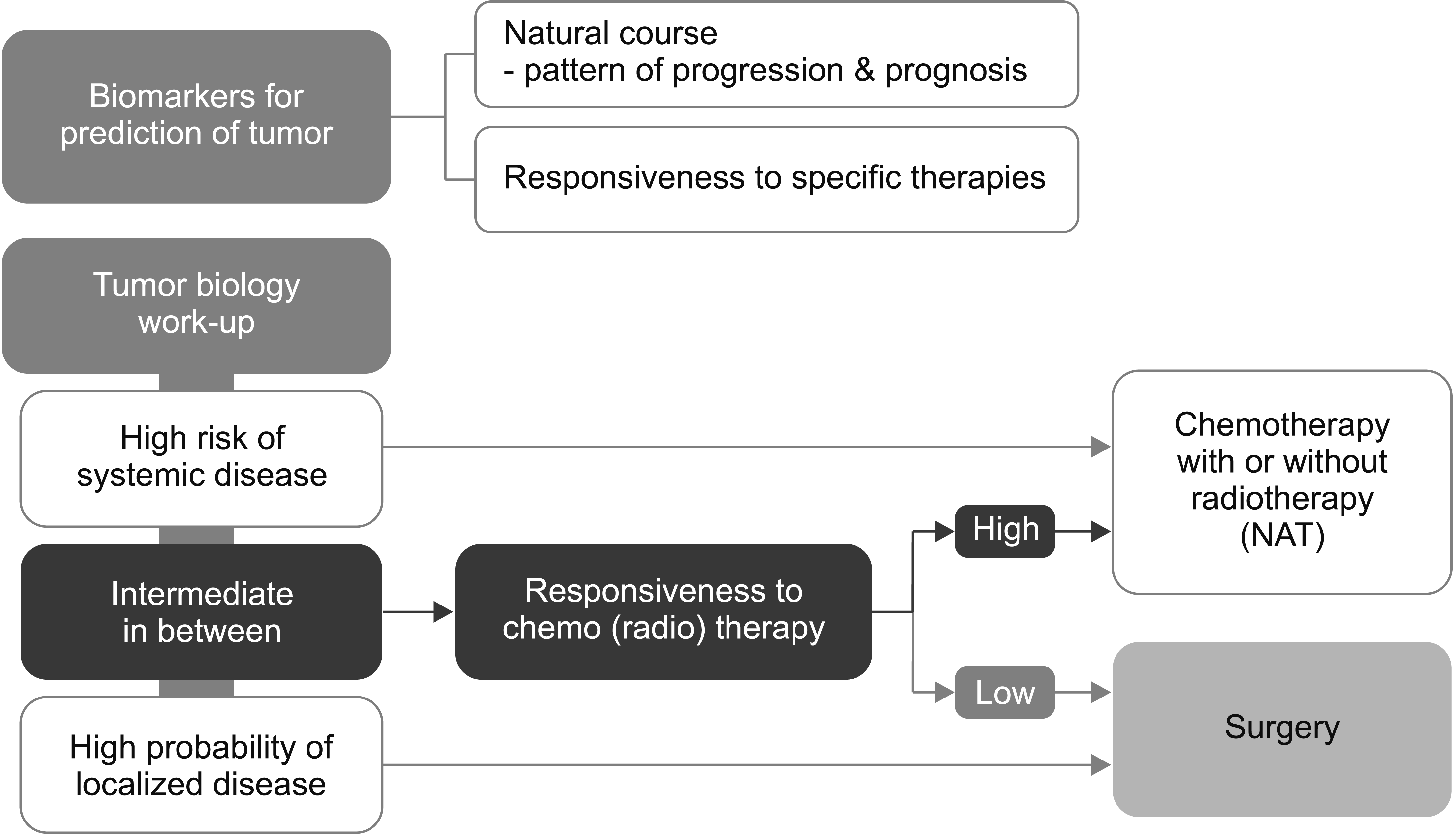
In addition to these factors, data on many potential non-morphological factors, including biological factors of the tumor and conditional factors of the host can be obtained preoperatively by different methods and tests using various biological samples. Tumor markers other than CA19-9, specific symptoms (such as pain, weight loss, and diabetes), various inflammation-based prognostic indices (such as the neutrophil-lymphocyte ratio [3], the platelet-lymphocyte ratio, the neutrophil-albumin ratio, and the C-reactive protein-albumin ratio), (modified) Glasgow score [3], information from the endoscopic ultrasonography biopsy (such as histological grading/differentiation and molecular or genetic alteration) [12], the germline mutation test [13], liquid biopsy modalities (including circulating tumor cells, circulating tumor DNA, cell-free DNA [cfDNA], miRNA, and methylated DNA) [14,15], potential imaging biomarkers, and radiomics via high-throughput extraction of image features [16] are being investigated. Kim et al. [17] suggested that a high KRAS gene mutation concentration and fractional abundance in cfDNA were poor prognostic markers that could guide therapeutic strategies in RPC. Conditional factors, such as age, Eastern Cooperative Oncology Group performance status [18], American Society of Anesthesiologists score, Charlson–Deyo comorbidity score [19], and various immuno-nutritional statuses, including sarcopenia and cachexia [20], are also relevant to the prognosis (Fig. 2).
Many factors determined by the tumor or host characteristics will be found to have prognostic significance in the near future. The issue is how the criteria or factors should be selected and combined from these attributes and variables to determine a treatment strategy. There may be different approaches, including population-based approaches, prognostic stratification/staging systems, and personalized outcome-based approaches using prognostic prediction models.
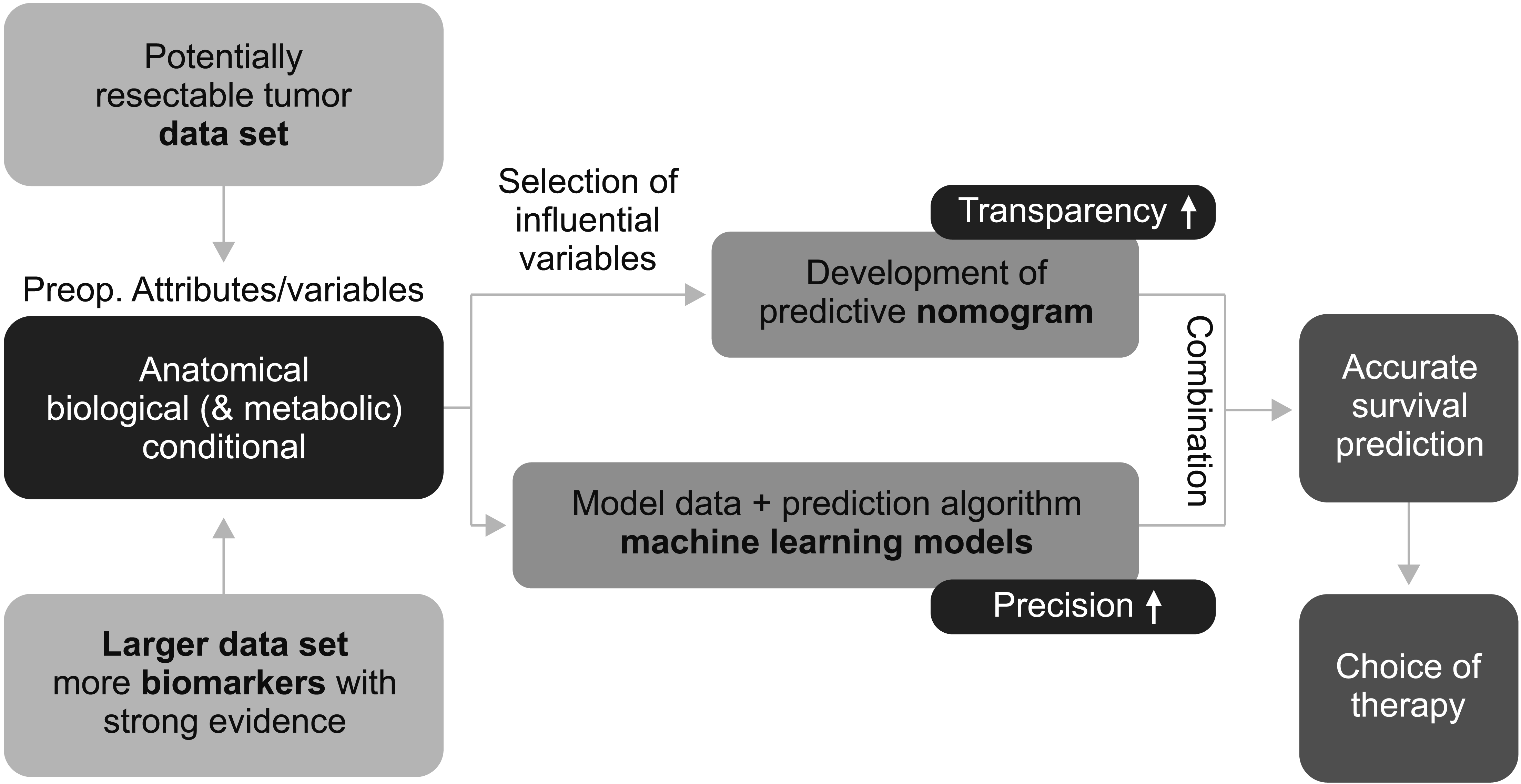
Once reliable biomarkers that predict the natural course, progression patterns, prognosis, and response to specific therapies are determined, cases can be stratified, and appropriate treatment options can be selected. For a personalized approach, large datasets of oncology patients can be used to develop predictive nomograms and machine learning models that can be used to predict surgical outcomes for individual patients and select the appropriate treatments (Fig. 3). The combination of the two predictive models will help make treatment decisions more transparent and accurate. Preoperative prognostic nomograms for postoperative recurrence or survival after surgery in patients with RPC have been developed and reported by several influential centers, suggesting that it is a “prognosis-based definition of resectability” and will be “a roadmap to new guidelines” in the era of precision oncology [12,19,21].
Go to : 
For RPC and BRPC, NAT is performed before surgery and, in most cases, surgery is performed after NAT. However, if the lesion progresses to unresectable disease despite NAT, surgery is not an option, and additional non-surgical treatment should be considered by changing the chemotherapeutic agents or adding other treatment modalities. Recently, a number of centers have been attempting surgery for LAPC that responds to chemotherapy and/or radiation therapy [22]. This is called conversion surgery or adjuvant surgery and is one of the most difficult treatment methods in PC management.
Recently, a series of studies on post-NAT surgery in advanced PC found that the prognosis was better when surgery was performed, suggesting that surgery should be considered in the absence of disease progression after NAT [8,23-25]. Byun et al. [26] reported that when surgery was performed in patients with advanced PC initially treated with FOLFIRINOX, the prognosis was better than continuing non-surgical treatment in the partial response/stable disease group.
However, reality can be quite different. Since surgical indications for LAPC after NAT have not been established thus far, some centers, including Heidelberg University, have aggressively accumulated surgical experience in LAPC [8]. However, most surgeons are still reluctant to perform surgery for LAPC, even if there is a response to non-surgical therapy. An international survey report revealed that there were significant differences in the management preferences of LAPC, including the proportion of surgeons recommending NAT and favoring surgical exploration after NAT [22]. The results suggest that the concept of resectability is evolving [22].
Surgery may be acceptable if there is a significant response to NAT. Therefore, standardized indications for conversion surgery are required. To obtain objective response criteria for NAT, the Response Evaluation Criteria in Solid Tumor (RECIST) as morphological criteria [27], various CA19-9 cutoff values as biological criteria [2], and the European Organization for Research and Treatment of Cancer (EORTC)/PET Response Criteria in Solid Tumors (PERCIST) criteria as metabolic criteria [28,29] can be used. Regarding CA19-9, some have argued for the need to identify patient groups who would benefit from surgery based on CA19-9 response levels after NAT, while others have agreed that stable disease on imaging is sufficient if there is any decrease in CA19-9 levels [30-34]. However, the target value varies from CA19-9 levels normalized to 80–400 U/mL or a 30%–80% reduction compared to the initial status [2]. The NCCN guidelines suggest resection after NAT in LAPC as one option when the CA19-9 level is reduced by > 50%, and there is clinical improvement [1]. The metabolic response determined using PET-SUV is another criterion used to quantify the response to NAT. The EORTC defined partial metabolic response as a reduction of > 25% in the SUV after more than one treatment cycle and complete metabolic response as the complete resolution of 18F-FDG uptake within the tumor volume [29]. In the PERCIST criteria, > 30% and a 0.8-unit decline in peak SUV lean (SUL, percentage reduction in the SUV) are required to define a response to treatment [28]. The aforementioned criteria can be combined to establish the criteria for resection. Moreover, the predicted outcome-based criteria can be adopted after NAT. In addition to the criteria used at diagnosis, the degree of objective response to NAT must be considered, and prognostic scoring systems, predictive nomograms, and machine learning models can be developed together.
In conclusion, the concept of resectability is shifting toward selecting surgery as a treatment strategy based on personalized surgical outcome predictions. Biomarkers predicting surgical outcomes should be further investigated and developed for rational treatment strategy selection. Response data from NAT-related factors should be collected to establish objective response criteria and surgical indications. Multicenter collaboration is needed to develop a large database with standardized data collection and establish more accurate outcome prediction models and new resectability criteria.
Go to : 
ACKNOWLEDGEMENTS
Figure development was supported by the Creative Media Service in National Cancer Center Korea.
Go to : 
Notes
AUTHOR CONTRIBUTIONS
Conceptualization: KSW. Data curation: All authors. Methodology: All authors. Visualization: All authors. Writing - original draft: All authors. Writing - review & editing: All authors.
Go to : 
REFERENCES
1. National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology: pancreatic adenocarcinoma, version 2 [Internet]. Plymouth Meeting: NCCN 2021. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1455. cited 2021 Jun 5. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1455.
2. Ishido K, Hakamada K, Kimura N, Miura T, Wakiya T. Essential updates 2018/2019: current topics in the surgical treatment of pancreatic ductal adenocarcinoma. Ann Gastroenterol Surg. 2020; 5:7–23. DOI: 10.1002/ags3.12379. PMID: 33532676. PMCID: PMC7832965.

3. Bockhorn M, Uzunoglu FG, Adham M, Imrie C, Milicevic M, Sandberg AA, et al. 2014; Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 155:977–988. DOI: 10.1016/j.surg.2014.02.001. PMID: 24856119.

4. Cady B. 1997; Basic principles in surgical oncology. Arch Surg. 132:338–346. DOI: 10.1001/archsurg.1997.01430280012001. PMID: 9108752.

5. Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, et al. 2018; FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 379:2395–2406. DOI: 10.1056/NEJMoa1809775. PMID: 30575490.

6. Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, et al. 2017; Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 389:1011–1024. DOI: 10.1016/S0140-6736(16)32409-6. PMID: 28129987.

7. Jang JY, Han Y, Lee H, Kim SW, Kwon W, Lee KH, et al. 2018; Oncological benefits of neoadjuvant chemoradiation with gemcitabine versus upfront surgery in patients with borderline resectable pancreatic cancer: a prospective, randomized, open-label, multicenter phase 2/3 trial. Ann Surg. 268:215–222. DOI: 10.1097/SLA.0000000000002705. PMID: 29462005.
8. Klaiber U, Schnaidt ES, Hinz U, Gaida MM, Heger U, Hank T, et al. 2021; Prognostic factors of survival after neoadjuvant treatment and resection for initially unresectable pancreatic cancer. Ann Surg. 273:154–162. DOI: 10.1097/SLA.0000000000003270. PMID: 30921051.

9. Kato H, Horiguchi A, Ito M, Asano Y, Arakawa S. Essential updates 2019/2020: multimodal treatment of localized pancreatic adenocarcinoma: current topics and updates in survival outcomes and prognostic factors. Ann Gastroenterol Surg. 2021; 5:132–151. DOI: 10.1002/ags3.12427. PMID: 33860134. PMCID: PMC8034700.

10. Isaji S, Mizuno S, Windsor JA, Bassi C, Fernández-Del Castillo C, Hackert T, et al. 2018; International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology. 18:2–11. DOI: 10.1016/j.pan.2017.11.011. PMID: 29191513.

11. Moon D, Kim H, Han Y, Byun Y, Choi Y, Kang J, et al. Preoperative carbohydrate antigen 19-9 and standard uptake value of positron emission tomography-computed tomography as prognostic markers in patients with pancreatic ductal adenocarcinoma. J Hepatobiliary Pancreat Sci. https://doi.org/10.1002/jhbp.845 [in press]. 2020; DOI: 10.1002/jhbp.845. PMID: 33063453.

12. Dreyer SB, Pinese M, Jamieson NB, Scarlett CJ, Colvin EK, Pajic M, et al. 2020; Precision oncology in surgery: patient selection for operable pancreatic cancer. Ann Surg. 272:366–376. DOI: 10.1097/SLA.0000000000003143. PMID: 32675551. PMCID: PMC7373491.
13. Hu H, Zhu Y, Pu N, Burkhart RA, Burns W, Laheru D, et al. 2020; Association of germline variants in human DNA damage repair genes and response to adjuvant chemotherapy in resected pancreatic ductal adenocarcinoma. J Am Coll Surg. 231:527–535.e14. DOI: 10.1016/j.jamcollsurg.2020.06.019. PMID: 32659497.

14. Gemenetzis G, Groot VP, Yu J, Ding D, Teinor JA, Javed AA, et al. 2018; Circulating tumor cells dynamics in pancreatic adenocarcinoma correlate with disease status: results of the prospective CLUSTER study. Ann Surg. 268:408–420. DOI: 10.1097/SLA.0000000000002925. PMID: 30080739.

15. Heredia-Soto V, Rodríguez-Salas N, Feliu J. 2021; Liquid biopsy in pancreatic cancer: are we ready to apply it in the clinical practice? Cancers (Basel). 13:1986. DOI: 10.3390/cancers13081986. PMID: 33924143. PMCID: PMC8074327.

16. Abunahel BM, Pontre B, Kumar H, Petrov MS. 2021; Pancreas image mining: a systematic review of radiomics. Eur Radiol. 31:3447–3467. DOI: 10.1007/s00330-020-07376-6. PMID: 33151391.

17. Kim MK, Woo SM, Park B, Yoon KA, Kim YH, Joo J, et al. 2018; Prognostic implications of multiplex detection of KRAS mutations in Cell-Free DNA from patients with pancreatic ductal adenocarcinoma. Clin Chem. 64:726–734. DOI: 10.1373/clinchem.2017.283721. PMID: 29352043.
18. Tas F, Sen F, Odabas H, Kılıc L, Keskın S, Yıldız I. 2013; Performance status of patients is the major prognostic factor at all stages of pancreatic cancer. Int J Clin Oncol. 18:839–846. DOI: 10.1007/s10147-012-0474-9. PMID: 22996141.

19. Oba A, Croce C, Hosokawa P, Meguid C, Torphy RJ, Al-Musawi MH, et al. Prognosis based definition of resectability in pancreatic cancer: a road map to new guidelines. https://doi.org/10.1097/SLA.0000000000003859 [in press]. Ann Surg. 2020; DOI: 10.1097/SLA.0000000000003859. PMID: 32149822.

20. Ozola Zalite I, Zykus R, Francisco Gonzalez M, Saygili F, Pukitis A, Gaujoux S, et al. 2015; Influence of cachexia and sarcopenia on survival in pancreatic ductal adenocarcinoma: a systematic review. Pancreatology. 15:19–24. DOI: 10.1016/j.pan.2014.11.006. PMID: 25524484.

21. Kim N, Han IW, Ryu Y, Hwang DW, Heo JS, Choi DW, et al. 2020; Predictive nomogram for early recurrence after pancreatectomy in resectable pancreatic cancer: risk classification using preoperative clinicopathologic factors. Cancers (Basel). 12:137. DOI: 10.3390/cancers12010137. PMID: 31935830. PMCID: PMC7016958.

22. Reames BN, Blair AB, Krell RW, Groot VP, Gemenetzis G, Padussis JC, et al. 2021; Management of locally advanced pancreatic cancer: results of an international survey of current practice. Ann Surg. 273:1173–1181. DOI: 10.1097/SLA.0000000000003568. PMID: 31449138.
23. Rangelova E, Wefer A, Persson S, Valente R, Tanaka K, Orsini N, et al. 2021; Surgery improves survival after neoadjuvant therapy for borderline and locally advanced pancreatic cancer: a single institution experience. Ann Surg. 273:579–586. DOI: 10.1097/SLA.0000000000003301. PMID: 30946073.
24. Michelakos T, Pergolini I, Castillo CF, Honselmann KC, Cai L, Deshpande V, et al. 2019; Predictors of resectability and survival in patients with borderline and locally advanced pancreatic cancer who underwent neoadjuvant treatment with FOLFIRINOX. Ann Surg. 269:733–740. DOI: 10.1097/SLA.0000000000002600. PMID: 29227344.

25. Gemenetzis G, Groot VP, Blair AB, Laheru DA, Zheng L, Narang AK, et al. 2019; Survival in locally advanced pancreatic cancer after neoadjuvant therapy and surgical resection. Ann Surg. 270:340–347. DOI: 10.1097/SLA.0000000000002753. PMID: 29596120. PMCID: PMC6985003.

26. Byun Y, Han Y, Kang JS, Choi YJ, Kim H, Kwon W, et al. 2019; Role of surgical resection in the era of FOLFIRINOX for advanced pancreatic cancer. J Hepatobiliary Pancreat Sci. 26:416–425. DOI: 10.1002/jhbp.648. PMID: 31218836.

27. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. 2009; New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. DOI: 10.1016/j.ejca.2008.10.026. PMID: 19097774.

28. Wahl RL, Jacene H, Kasamon Y, Lodge MA. 2009; From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 50 Suppl 1:122S–150S. DOI: 10.2967/jnumed.108.057307. PMID: 19403881. PMCID: PMC2755245.

29. Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, et al. 1999; Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET study group. Eur J Cancer. 35:1773–1782. DOI: 10.1016/S0959-8049(99)00229-4. PMID: 10673991.
30. Truty MJ, Kendrick ML, Nagorney DM, Smoot RL, Cleary SP, Graham RP, et al. 2021; Factors predicting response, perioperative outcomes, and survival following total neoadjuvant therapy for borderline/locally advanced pancreatic cancer. Ann Surg. 273:341–349. DOI: 10.1097/SLA.0000000000003284. PMID: 30946090.

31. Tsai S, George B, Wittmann D, Ritch PS, Krepline AN, Aldakkak M, et al. 2020; Importance of normalization of CA19-9 levels following neoadjuvant therapy in patients with localized pancreatic cancer. Ann Surg. 271:740–747. DOI: 10.1097/SLA.0000000000003049. PMID: 30312198.

32. Ushida Y, Inoue Y, Ito H, Oba A, Mise Y, Ono Y, et al. 2021; High CA19-9 level in resectable pancreatic cancer is a potential indication of neoadjuvant treatment. Pancreatology. 21:130–137. DOI: 10.1016/j.pan.2020.11.026. PMID: 33303373.

33. Ye C, Sadula A, Ren S, Guo X, Yuan M, Yuan C, et al. 2020; The prognostic value of CA19-9 response after neoadjuvant therapy in patients with pancreatic cancer: a systematic review and pooled analysis. Cancer Chemother Pharmacol. 86:731–740. DOI: 10.1007/s00280-020-04165-2. PMID: 33047181.

34. Liu H, Zenati MS, Rieser CJ, Al-Abbas A, Lee KK, Singhi AD, et al. 2020; CA19-9 change during neoadjuvant therapy may guide the need for additional adjuvant therapy following resected pancreatic cancer. Ann Surg Oncol. 27:3950–3960. DOI: 10.1245/s10434-020-08468-9. PMID: 32318949. PMCID: PMC7931260.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download